Alkanes-Paraffins-6: Hlogenation of Alkanes
Chemical properties
(i) Halogenation
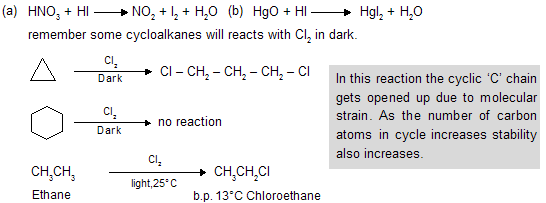
Depending upon which hydrogen atom is replaced, any of a number of isomeric products can be formed from a single alkane. Ethane can yield only one halo ethane ; propane, n-butane, and isobutane can yield two isomers each; n-pentane can yield three isomers, and isopentane, four isomers. Experiment has shown that on halogenation an alkane yields a mixture of all possible isomeric products, indicating that all hydrogen atoms are susceptible to replacement. For example, for chlorination :
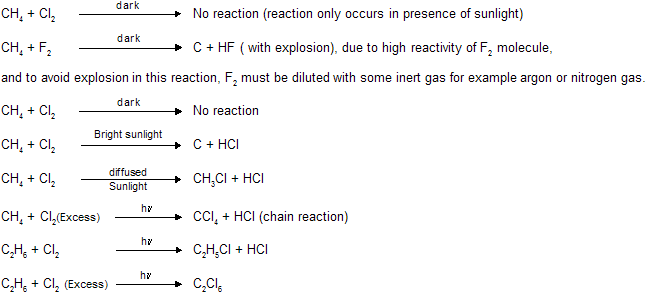
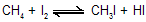
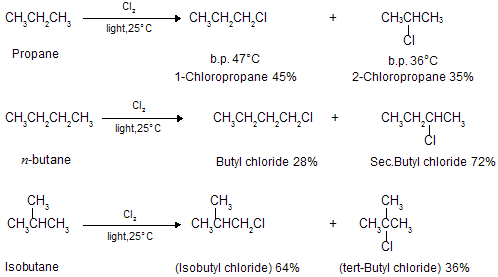
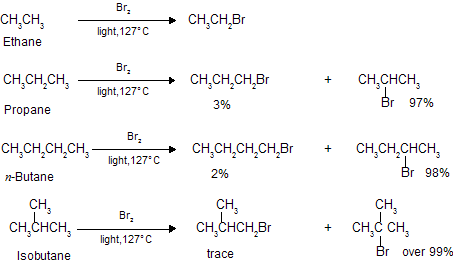
Although both chlorination and bromination yield mixtures of isomers, the results given above show that the relative amounts of the various isomers differ markedly depending upon the halogen used. Chlorination gives mixtures in which no isomer greatly predominates ; in bromination, by contrast, one isomer may predominate to such an extent as to be almost the only product, making up 97-99% of the total mixture. In bromination, there is a high degree of selectivity as to which hydrogen atoms are to be replaced.
Halogenation of alkanes is not suitable for the laboratory preparation of alkyl halides. In chlorination, any one product is necessarily formed in low yield, and is difficult to separate from its isomers, whose boiling points are seldom far from its own. Even bromination of alkanes is seldom used.
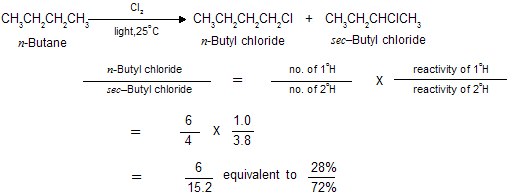
Chlorination of many alkanes has prove these typical results. After allowance is made for differences in the probability factor, the rate of abstraction of hydrogen atoms is always found to follow the sequence 3o > 2o > 1o. At room temperature, for example, the relative rates per hydrogen atom are 5.0 : 3.8 : 1.0. Using these values we can predict quite well the ratio of isomeric chlorinated products from a given alkane. For example :
The same sequence of reactivity, i.e., 3o > 2o > 1o, is found in bromination, but with enormously large reactivity ratios. At 127oC, for example, the relative rates per hydrogen atom are 1600 : 82 : 1. Here, differences in reactivity are marked as vastly to outweigh the probability factors.