Acid - Derivatives-4
When esters reacts with amines to form amides, reaction is known as aminolysis. Notice that the aminolysis of an ester requires only one equivalent of amine, unlike the aminolysis of an acyl halide or an acid anhydride, which we saw requires two equivalents. This is because the leaving group of an ester (RO-) is more basic than the amine nucleophile (RNH2), so the alkoxide ion rather than unreacted amine picks up the proton produced in the reaction.
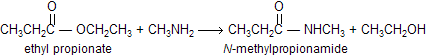
The tetrahedral intermediate formed when an ester is hydrolyzed has both an RO- leaving group and an HO- leaving group. Because both leaving groups have approximately the same basicity, they are equally likely to be eliminated.
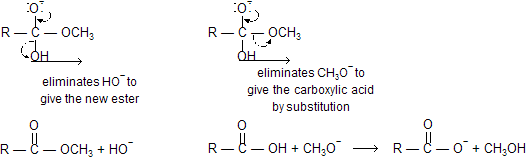
Notice that when CH3O- is eliminated, the final products are a carboxylate ion and methanol. If only one species is protonated, it will be the more basic one (CH3O- is more basic than RCOO-).
When ester reacts with an alcohol in the presence of acid to form a new ester and a new alcohol. This is called a transesterification reaction. Because both alcohols have approximately the same leaving tendency in the tetrahedral intermediate, an excess of the reactant alcohol is necessary to drive the equilibrium to the right. Or, if the boiling point of the product alcohol is significantly lower than the boiling points of the other reaction components, the reaction can be driven to the right by distilling off the product alcohol as it is formed. The rate of transesterification can be increased by H+ or by the conjugate base (RO-) of the reactant alcohol.
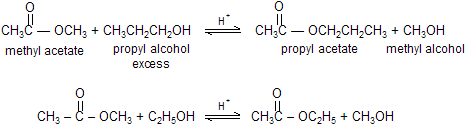
Because phenols are stronger acids than alcohols, phenolate ions (ArO-) are weaker bases than alkoxide ions (RO-). This means that phenyl esters are more reactive than alkyl esters.
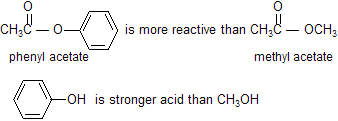
The reaction of an ester with an amine is also a slow reaction. However, unlike the reaction of an ester with water or an alcohol, the rate of the reaction of an ester with an amine cannot be increased by H+ or by HO- or RO-. Aminolysis of an ester can be driven to completion by using excess amine or by distilling off the alcohol as it is formed.
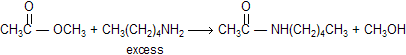
(iii) Claisen Ester Condensation
Esters which contains ![]() on reaction with a strong base, e.g., C2H5ONa or (CH3)3CONa undergo self-condensation by intermediate carbanian to produce
on reaction with a strong base, e.g., C2H5ONa or (CH3)3CONa undergo self-condensation by intermediate carbanian to produce ![]()
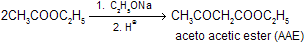
This reaction is called Claisen ester condensation although there are several closely related reactions which follow the same path.