Alcohol-Diol-Triol-1
Alcohols,diols and triols
Preparation
(1) Hydrolysis of alkyl halides This reaction is useful only with reactants that do not undergo E2 elimination readily. It is really used for the synthesis of alcohols where alkyl halides are primary in nature.
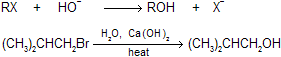 |
(2) Reaction of Grignard reagents with aldehydes and ketones A method that allows for alcohol preparation with formation of new carbon bonds. Primary, secondary, and tertiary alcohols can all be prepared and stepping up to two carbon atom also can takes place by the help of cyclic epoxides.
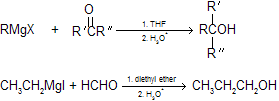 |
(3) Reaction of organolithium reagents with aldehydes and ketones Organolithium reagents react with aldehydes and ketones in a manner similar to that of Grignard reagents to form alcohols. Other reagents which are less reactive than alkyl lithium is dialkyl cadmium, R2Cd where active species is carbanian, and forms similar kind of product.
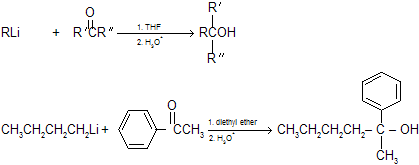 |
(4) Reaction of Grignard reagents with esters Produces tertiary alcohols in which two of the substituents on the hydroxyl- bearing carbon are derived from the Grignard reagent. If we want to stop this reaction at carbonyl compound we must use less reactive reagent like dialkyl cadmium R2Cd. Remember the alkyl group in ester goes out finally as alcohol.
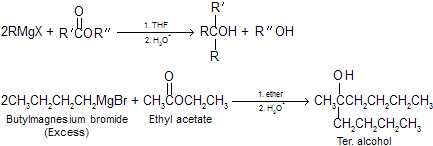 |
(5) Acid catalyzed hydration of alkenes The elements of water add to the double bond according to the markonikov’s rule, by intermediate carbocation so rearrangement occurs.
 |