Alcohol-Diol-Triol-6
(d) Catalytic dehydrogenation: It involves the passing of vapours of alcohol over reduced copper at 300°C and the product thus formed is identified.
(i) Primary alcohols give aldehydes (dehydrogenation).
(ii) Secondary alcohols give ketones (dehydrogenation).
(iii) Tertiary alcohols form olefins (alkenes). This is dehydration.
Manufacture of Ethyl Alcohol by Fermentation (Alcoholic Fermentation)
The conversion of sugar into ethyl alcohol by yeast is called alcoholic fermentation. In alcoholic fermentation cane sugar or glucose is the fermenting material. Therefore, any natural product which contains these sugars or can be easily converted into them, becomes a source of ethyl alcohol. The raw materials for alcohol industry are:
(a) Materials containing sugars like cane juice, beets, molasses and fruit juices. The cheapest source is molasses.
(b) Materials containing starch like potato, rice, barley and maize.
- From molasses: Molasses is a dark coloured syrupy mass left after the crushing of cane sugar or beet sugar crystals from the concentrated juice. It still contains 30% of uncrystallised sucrose and about 32% of invert sugar - a mixture of glucose and fructose. It forms an excellent cheap source of industrial ethyl alcohol. The various steps involved in the manufacture are:
(i) Dilution: The molasses is diluted with water until a concentration of 8 - 10% sugar is obtained in solution. A small amount of dilute sulphuric acid is added as to adjust the pH value of solution about 4-4.5. The acidity is favourable for the growth of yeast but unfavourable for the growth of undesirable bacteria. To this solution a small quantity of ammonium sulphate is added which acts as a food for the yeast cells.
(ii) Alcoholic fermentation The diluted solution is taken in big fermentation tanks and some yeast is added (5% by volume of the liquid). The mixture is allowed to stand for a few days. The temperature is kept about 30°C. Fermentation sets in and the enzyme invertase converts sucrose into glucose and fructose which are further converted into ethyl alcohol by another enzyme, zymase.
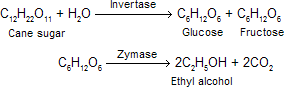
The fermentation is complete in three days and carbon dioxide is the by-product. The fermented liquor is filtered.
(iii) Fractional distillation The fermented liquor is technically called as wash or wort. The approximate composition of wash is: 6-10% ethyl alcohol, 3-5% glycerol, higher alcohols (fusel oil), acetaldehyde, etc. Wash is subjected to fractional distillation in a coffee still. Each coffee still consists of two fractionating columns known as analyser and rectifier which are provided with perforated plates. It works on counter-current principle.
The wash is preheated by circulating it through the coil round the rectifier and then introduced into the analyser. A current of steam is passed from the bottom of the analyser where by the alcohol and other volatile constituents present in the wash rise up along with steam and enter the rectifier. In the upward passage steam goes on condensing while the vapours of alcohol leaving from the top of the rectifier are condensed and collected. This is know as row spirit and contains about 90% alcohol. The liquid collected at the bottom of analyser is called spent wash.
(iv) Rectification Ethyl alcohol obtained above contains in addition to water various other impurities which are removed by careful fractional distillation. The following fractions are obtained:
Primary fraction - It contains low boiling liquids like acetaldehyde.
Secondary fraction - It contains 93-95% ethyl alcohol and is called rectified spirit.
Last fraction - It contains water and fusel oil. Fusel oil is a mixture of n-propyl, n-butyl, n-amyl, isoamyl and optically active amyl alcohols.
2. From starch: The manufacture of ethyl alcohol from starchy materials like potatoes, barley, maize, etc., involves the following steps:
(i) Saccharification: The starch is first converted into maltose. The process of conversion is known as saccharification. It completes in three steps:
(a) Mashing : The process of liberating starch from starchy materials is called mashing .The starchy material is treated with super heated steam as to break the cell walls and to form a paste like mass called mash.
(b) Malting : Moist barley is allowed to germinate in dark at 15°C. Germinated barley called malt is heated to 60°C to stop the further growth. It is crushed and extracted with water. It is filtered. The filtrate containing diastase enzyme is known as malt extract.
(c) Hydrolysis : Malt extract is added to mash at 50-60°C. The enzyme diastase hydrolyses the starch into maltose in about half an hour’s time.
(ii) Alcoholic fermentation: The hydrolysed starch solution containing maltose is then fermented by yeast for 2-3 days at 30°C. The enzyme maltase present in the yeast hydrolyses maltose into glucose. The other enzyme, zymase present in the yeast converts glucose to ethyl alcohol.
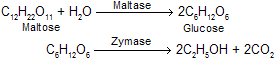
(iii) Distillation and rectification of the fermented liquor: This is done in a similar way as described in the first method.
By-products of alcohol industry: The important by-products obtained during manufacture of ethyl alcohol are:
(a) Carbon di oxide : Large quantities of carbon dioxide are evolved during fermentation. It is usually solidified and sold as dry ice.
(b) Acetaldehyde : During rectification, acetaldehyde is obtained in the first fraction.
(c) Fusel oil : It is obtained in the last fraction of the rectification step. It is used for the preparation of amyl alcohols and amyl acetate.
(d) Spent wash : This is the residue left in the still after distillation. It contains nitrous matter and is used as cattle feed.
(e) Argol (potassium hydrogen tartrate) : This separates out as a crust during fermentation. It consists of crude potassium hydrogen tartrate which is used to manufacture tartaric acid.