Alcohol-Diol-Triol-7
Preparation of Absolute Alcohol
The rectified spirit contains 95.5% ethyl alcohol and 4.5% water. It is a constant boiling mixture and water cannot be removed simply by distillation. Hence some special methods are adopted for removing water from rectified spirit as to obtain 100% ethyl alcohol, i.e., absolute alcohol.
Laboratory method: Rectified spirit is kept in contact with quick lime (CaO) for a day and then distilled over quick lime. Final traces of water are removed by adding anhydrous copper sulphate. It is again distilled to get absolute alcohol.
Industrial method: This method is based on azeotropic distillation. Excess of benzene is added to the rectified spirit. Benzene forms a ternary mixture with water and alcohol which distills at 64.8°C till whole of the water is removed.

The remaining benzene then forms another low boiling binary mixture which distills over at 68.2°C till pure alcohol is left behind.
The absolute alcohol boils at 78.3°C.
Methylated spirit or denatured alcohol: In order to make the industrial alcohol unfit for drinking purposes, the rectified alcohol is denatured by adding various poisonous compounds. In most of the countries, the denaturing is done by adding 5 to 10% of methyl alcohol and 0.5% rubber distillate (caoutchoucine ) and 0.5% pyridine bases. This denatured spirit is sold in the market under the name methylated spirit.
Power alcohol: Like petrol, absolute ethyl alcohol can be used in internal combustion engines to derive power. But ethyl alcohol alone is not sufficiently volatile and is thus generally mixed with other volatile combustible substances like petrol, benzene, etc. When alcohol is used in this form, it is known as power alcohol. Since rectified spirit does not mix with petrol, absolute alcohol is used for this purpose, A mixture containing 25% absolute alcohol and 75% petrol acts as a good fuel for motor cars and is sold in the market as power alcohol.
Alcoholic Beverages and alcoholometry
The colour, flavour and taste of a particular beverage depends on the materials used, process of fermentation and subsequent treatments. The following list includes some common beverages with their alcoholic content and source form which it is obtained.
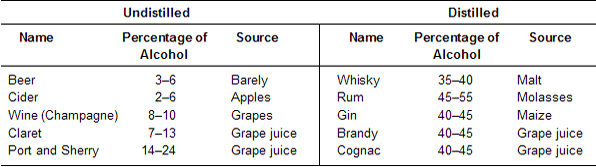
Alcoholic beverages when taken in small quantity act as stimulants but when taken regularly in excess, may decrease the energy of the body, retard the mental powers and damage the liver causing cirrhosis of liver.
The process of determining the percentage of alcohol in a given sample is known as alcoholometry. This is done by measuring the specific gravity of the sample with the help of hydrometer and then reading the percentage of alcohol against this value of specific gravity from a standard reference table.
An alcohol-water mixture having specific gravity 0.91976 at 15°C and containing 57.1% of ethyl alcohol by volume or 49.3% by weight is called proof-spirit. A sample having higher percentage of ethyl alcohol in comparison to proof-spirit is referred to as over-proof (O.P.) and the one having lower alcohol content than proof-spirit is known as under-proof (U.P). Thus 15 U.P. means that 100 ml. of the sample contains as much alcohol as 85 ml. of proof spirit. Similarly, 15 OP means that 100 ml. of the sample contains as much of alcohol as 115 ml. of proof spirit.
Glycerol or glycerine, HOCH2CH(OH)CH2OH
Glycerol is a trihydric alcohol. It may be considered as derivative of propane, obtained by replacement of three hydrogen atoms from different carbon atoms by three hydroxyl group. In industry, its known as glycerine.
Manufacture
(i) From fats and oil: On hydrolysis of fats and oils, glycerol and higher fatty acids are formed.
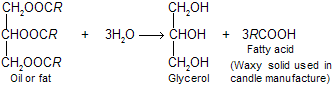
The hydrolysis is carried out either by alkali solution (Lye) or by super heated steam.
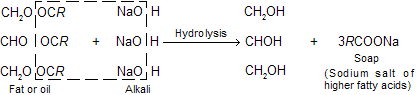
In the manufacture of soap (sodium salt of higher fatty acids) and in the manufacture of fatty acids required in candle industry, glycerol is obtained as by-product.
(ii) By fermentation of sugars: During alcoholic fermentation of sugar about 3% glycerol is formed. However, if the fermentation is done in presence of sodium sulphite, the yield can be increased to 25%.
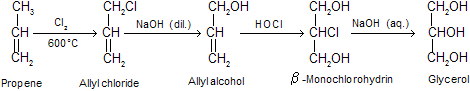
(iii) Synthesis (from propene): Today much of glycerol is obtained from propene (a product of catalytic cracking of petroleum).
Physical properties
(i) It is a colourless, odourless, viscous and hygroscopic liquid, sweet in taste and non-toxic in nature.
(ii) It is soluble in water and ethyl alcohol but insoluble in ether.
(iii) It has high boiling point, i.e., 290°C. The high viscosity and high boiling point of glycerol are due to association through hydrogen bonding purified in the lab by reduced pressure distillation or vacuum distillation.
Chemical properties
Glycerol molecule contains two primary – OH groups and one secondary – OH group. Thus, it shows characteristics of both primary and secondary alcohols. The carbon atoms in glycerol are indicated as![]()
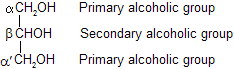
In general, primary – OH groups are more reactive than secondary – OH group.
(i) Reaction with sodium: Only primary alcoholic groups are attacked one by one and secondary alcoholic group is not attacked, Sodium forms monosodium glycerolate at room temperature and disodium glycerolate at higher temperature.
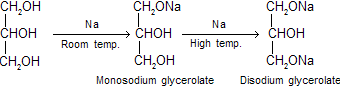
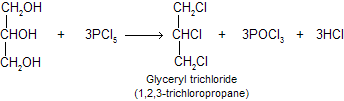
(ii) Reaction with PCI5: All three OH groups are replaced by CI atoms.