Alcohol-Diol-Triol-9
(ix) Oxidation: Glycerol gives different oxidation products depending on the nature of oxidising agent. The following products may be obtain during oxidation of glycerol.
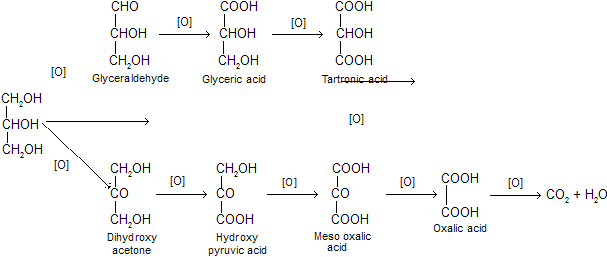
(a) Dilute HNO3 gives mainly glyceric acid.
(b) Conc. HNO3 oxidises glycerol into glyceric acid and tartronic acid.
(c) Bismuth nitrate gives mainly meso oxalic acid.
(d) Fenton’s reagent (H2O2 + FeSO4) or NaOBr or Br2- water in presence of Na2CO3 oxidises glycerol into a mixture of glyceraldehyde and dihydroxy acetone (or glycerose).
(e) With solid KMnO4, glycerol is oxidised to oxalic acid and carbon dioxide. The reaction is violent and explosion occurs.
Uses: Glycerol is used:
(a) As a sweetening agent in confectionery, beverages and medicines being nontoxic in nature.
(b) As antifreeze in automobile radiators.
(c) In the preparation of good quality of soap, hand lotions, shaving creams and tooth pastes.
(d) As a lubricant in watches and preservative.
Analytical Tests of Glycerol
(i) Acrolein test: When glycerol is heated with potassium hydrogen sulphate a very offensive odour is produced due to formation of acrolein. Its aqueous solution restores the colour of Schiff’s reagent and reduces Fehling’s solution and Tollen’s reagent.
(ii) Dunstan’s test: A drop of phenolphthalein is added to approximately 6 ml. of borax solution. The pink colour reappears on heating and disappears on cooling again.
(iii) To an equimolar mixture of phenol and glycerol, few drops of conc. H2SO4 are added and heated to 130°C. It is then boiled by adding water. Addition of ammonium hydroxide to this solution produces red colour.