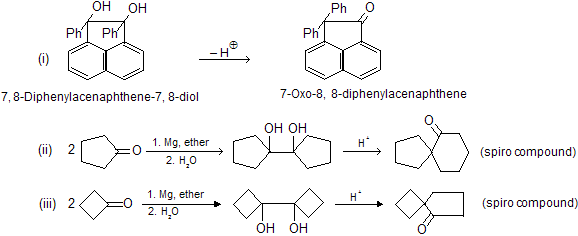
Aldehydes-Ketones-4
Mechanism of Cannizaro’s of benzaldehyde
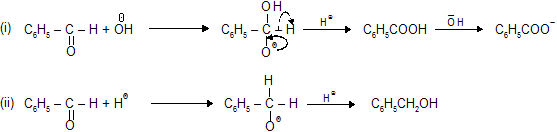
(b) Cross Cannizaro's Reaction
Reaction of carbonyl compounds (does not having
Whenever HCHO is present, there are only two main products otherwise there will be four products in all other cases. Because HCHO contains maximum reducing hydrogen atom so easily undergo oxidation process.
One of the most important applications is the crossed Cannizzaro reaction between formaldehyde and other aldehydes containing
(iv) Bimolecular Reduction or Pinacol Reaction
Two molecules of acetone undergo reduction in the presence of Mg/Hg to form Pinacol. Upon treatment with mineral acids, 2,3-dimethyl 2,3- butane diol (pinacol) is converted into methyl ter-butyl ketone (pinacolone). The 1,2-diol undergo dehydration in such a way that rearrangement of the carbon skeleton occurs. Other 1,2 diols undergo analogous reactions, which are known as pinacol pinacolone type rearrangement.
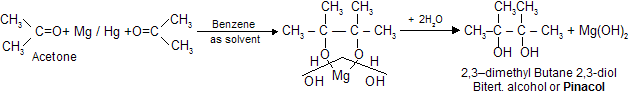
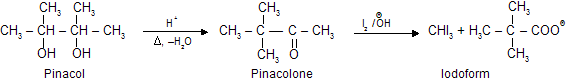
Mechanism
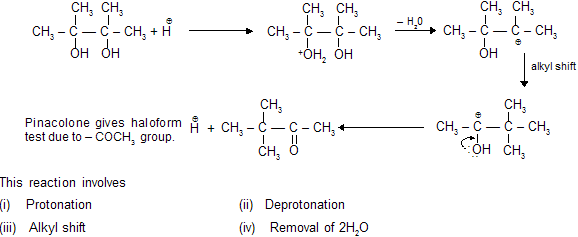
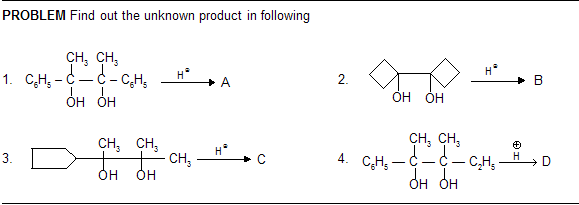
Pinacol–Pinacolone type Rearrangement
As the migrating group migrates with its electron pair, the more nucleophilic group might be expected to migrate. Thus, the order of migratory attitude amongst the aryl groups is p-anisyl > p-tolyl > phenyl > p-chlorophenyl, etc.
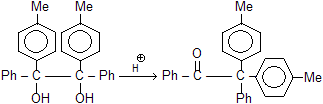
Remember, electron-attracting groups will retard the migration. The migratory aptitude amongst the alkyl groups is Me3C > Me2CH > Me. However, the stability of the initially formed carbocation may offset the migratory attitude order. Thus, in the compound 1, 1-dimethyl-2, 2-diphenyl glycol, the resonance-stabilized carbocation (I) is formed instead of (II) and so it is the methyl group and not the phenyl group which migrates, contrary to the above sequence.
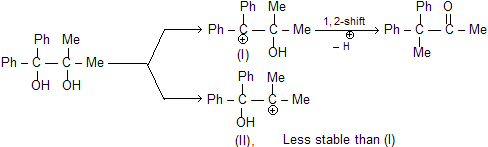
Steric hinderance may affect the rate of migration–p-anisyl group migrates 1000 times faster than o-anisyl group.
Migrating group attacks from the trans side or back side of to the leaving group. This has significant aspect in cyclic systems. Thus, the two isomers of 1, 2-dimethyl-cyclohexane-1, 2-diol give different products due to different orientations of the methyl and hydroxyl groups. The one (III) in which the Me and OH groups are trans to each other gives 2, 2-dimethylcyclohexanone by methyl shift. The other (IV) in which the Me and OH groups are cis to each other undergoes ring methylene group shift instead of Me-shift with consequent ring contraction to give 1-acetyl-1-methylcyclopentane (V).
Applications
Ketones from cyclic diols Pinacol rearrangement has been also applied to prepare ketones which are very difficult to prepare by other method.
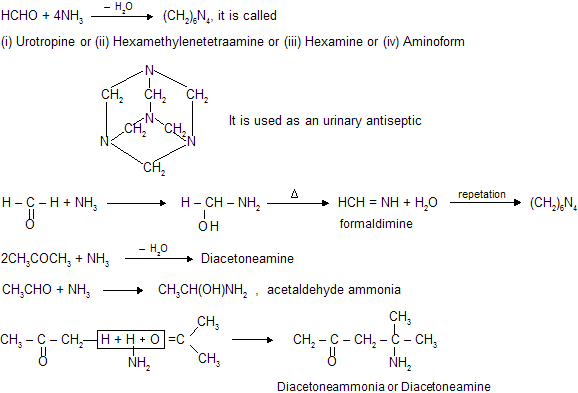
(v) Reaction with NH3
When carbonyl compounds reacts with ammonia, nucleophilic addition reaction occurs but products are formed according to praportions between ammonia and carbonyl compound. For example
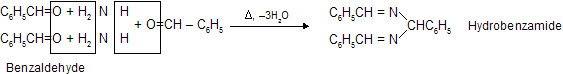 |
(vi) Reaction with Ammonia Derivatives NH2 – Z is an example of nucleophilic addition elemination reaction where – Z groups are following in nature. First attack from nucleophilic site of nitrogen atom to electrophilic carbon atom then elimination of water occurs simultaneously.
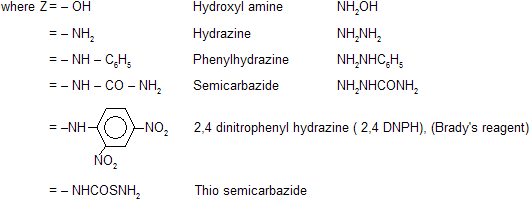
These reactions are usually carried out in weakly acidic medium because weak acid catalyses the reaction by protonating carbonyl oxygen but in presence of excess of acid nucleophile is also protonated which reduces its reactivity, so optimum pH is necessary. When zine compounds reacts with carbonyl compound they form crystalline solid as zone derivatives.
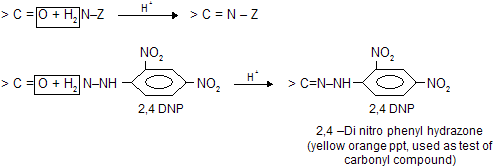
The reaction with 2,4–DNPH is used for sepration of carbonyl compound with noncarbonyl compound.
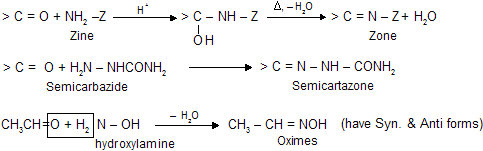
Always remember, presence of traces of