Aldehydes-Ketones-5
(vii) Distinction between aldehydes & Ketones
Aldehydes having reducing hydrogen atom whereas ketones don’t, thus only aldehydes reacts with oxidising agent and forms respective product.
(a) Ammonical AgNO3 (Tollen’s reagent)
Aldehydes reacts with silveroxide to form white precipitate of metallic silver while ketones cannot, due to absence of reducing hydrogen atom.
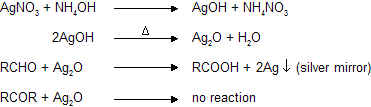 |
(b) Reaction with HgCl2 (Mercuric chloride)
Aldehydes reacts with mercuric chloride to form white precipitate of mercurous chloride which changes into black precipitate of metallic mercury.
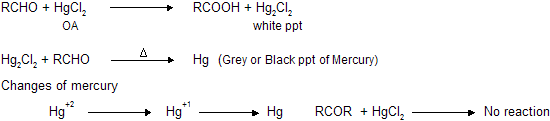 |
(c) Fehling's solution
There are two solutions which contains cupric oxide as oxidising agent, when reacts with aldehydes it forms red precipitate of cuprous oxide.
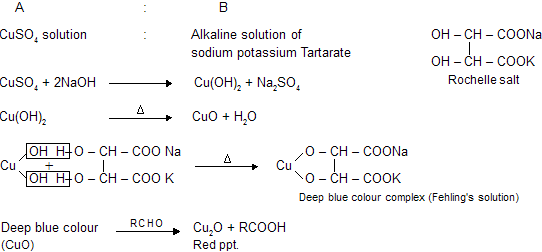
C6H5CHO and their aromatic derivatives do not give test with Fehling's solution because aromatic aldehydes are not good reducing agents. It gives reaction with tollen's reagent due to more oxidising nature of Ag2O than CuO. (Ag contains higher reduction potential than copper in electrochemical series)
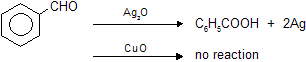 |
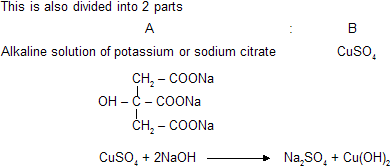
(d) Benedict's Solution
Similar chemical reaction and cupric oxide are present in benedict's solution but presence of citrate gives differant complex. It reacts with aldehydes to form red precipitate of Cu2O. It cannot reacts with benzaldehyde and its aromatic derivatives.
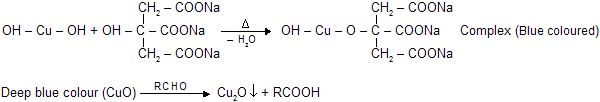
(e) Schiff’s Reagent
When dilute solution of p-rosaniline hydrochloride, pink in colour, passed through sulphurdioxide gas forms colourless solution known as schiff's reagent. This restore it colour by reducing nature of aldehyde while ketones gives no response.
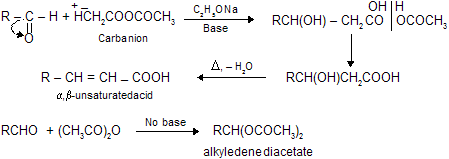
(viii) Reformatsky Reaction
When an
When, a mixture of the carbonyl compound,
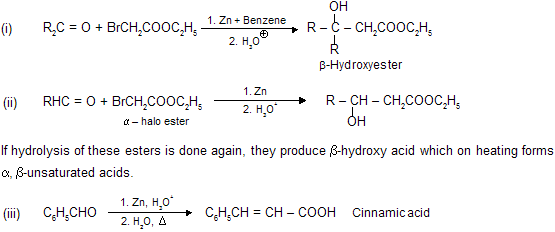
Aldol condensation, Knovengel’s reaction, Perkin reaction and Reformatsky reaction are base catalysed reaction so these reactions are carbanian active process. The advantage of using zinc in place of magnesium is that the organo-zinc compounds are less reactive than the organo-magnesium derivatives of
(ix) Perkin synthesis
In Perkin reaction, synthesis has been effected between aromatic aldehydes and aliphatic acid anhydrides in the presence of sodium or potassium salt of the acid corresponding to the anhydride, to yield
In this reaction active species also comes in the presence of base as carbanion (C– H2COOCOCH3).
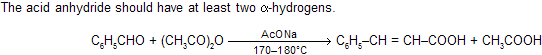
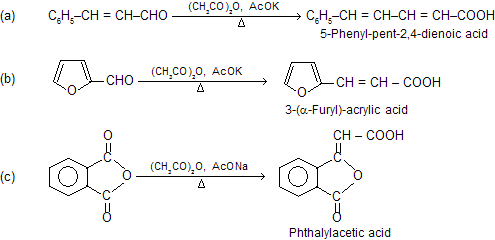
Besides simple aromatic aldehydes, their vinyl derivatives, heterocyclic aldehydes and even phathalic anhydride (as the carbonyl component) give this reaction.
When carbonyl compounds reacts with acetic anhydride in the presence of base to form active carbanian species, which give nucleophilic addition with carbonyl compounds. But remember, absence of base gives simple fission of anhydride (no carbanian) which on reaction with carbonyl forms stable alkyledene acetates.
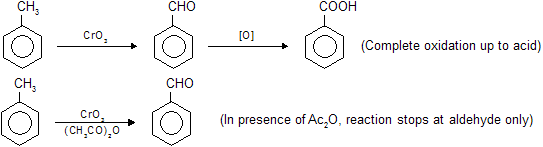
Toluene oxidation by chromic acid forms benzoic acid while in the presence of acetic anhydride reaction stops at benzaldehyde due to formation of stable intermediate benzeledene di-acetate which on hydrolysis again form –CHO group. So protection of –CH3 group oxidation at –CHO group, we use chromic acid and anhydride mixture. Following reactions are given below