Aldehydes-Ketones-6
(x) Witting reaction
Witting reaction gives an important and useful method for the preparation of alkenes by the reaction of aldehydes or ketones with alkylidenetriphenylphosphorane (Ph3P = CR2) or simply called as phosphorane.
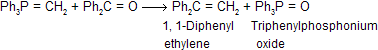
The Witting reagent, alkylidenetriphenylphosphorane, is prepared by reaction of trialkyl or triarylposphine usually the latter with an alkyl halide in ether solution. Finally resulting phosphonium salt is reacted with a strong base (such as C6H5Li, BuLi, NaNH2, NaH, C2H5ONa, etc.) which removes a haloacid to give the reagent, methylenetriphenyl phosphorane (II).
In end, carbonyl compound is directly treated with the ethernal solution of the above reagent to form many compounds.
Mechanism
The reaction go through by the nucleophilic attack of the ylide on the carbonyl carbon. The dipolar complex (betain) so formed undergo electronic exchange decomposes to olefin and triphenylphosphine oxide through a four-centred transition state.
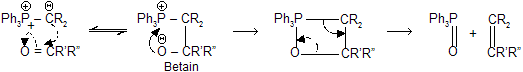
The mechanism is strongly supported by an example that an optically active phosphonium salt reacts to produce a phosphine oxide with retention of configuration in the final product.
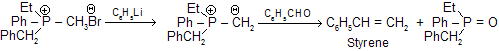
(xi) Lederer Manasse’s Reaction
When phenol is treated with 40% aqueous solution of formaldehyde (formalin) in the presence of a dilute acid or alkali at low temperature, a mixture of o-and p-hydroxy benzyl alcohol is formed.
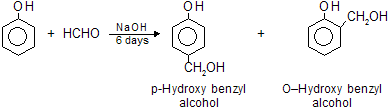
This reaction is called Lederer-Manasse reaction. On heating for a short time, these compounds unergo condensation reaction with themselves and unchanged phenol and give linear polymers by elimination of water.
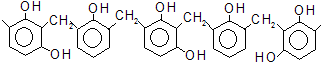
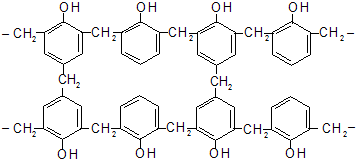
These reactions are the basis of the preparation of phenol formaldehyde resins. These materials were developed by Backland and are hence called bakelite. They are thermoplastic solids soluble in many organic solvents. When warmed with hexa methylene tetramine. (CH2)6N4 , which splits up to formaldehyde and ammonia, further methylene bridges are formed and a three-dimensional polymer results.
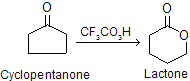
(xii) Baeyer–villiger rearrangement
Baeyer--Villiger rearrangement is an example of the migration of a group from carbon to electron-deficient oxygen.The reaction first involves the oxidation of ketones to esters by the treatment with peracids such as peracetic acid, performic acid, meta chloroperbenzoic acid (MCPBA), perbenzoic acid, pertrifluoroacetic acid, permonosulphuric acid, etc. This reaction can also be done by H2O2 and base.
Cyclic ketones are converted to lactones with expansion of ring.
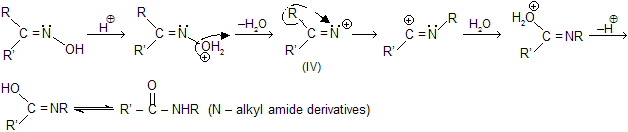
(xiii) Beckmann rearrangement
The acid-catalyzed conversion of ketoximes to N-substituted amides is known as Beckmann rearrangement. The reaction is catalysed by acidic reagents such as, H2SO4, SOCl2, P2O5, PCl5, Al2O3C6H5SO2Cl, H3PO4 and many others.
The reaction proceeds by the migration of a group from carbon to electron-deficient nitrogen.
Some aldoximes undergo the rearrangement process in the presence of polyphosphoric acid (PPA) but the reaction is not a general one. The migration of the group not depends on the migrational activity but upon the orientation of the group in relation to the OH group. It is found that the migrating group is always anti (i.e., trans) to the hydroxyl group. So we can say that, the reaction is stereospecific.
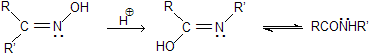
Mechanism of reaction
(xiv) Benzilic acid rearrangement (modified intra molecular cannizaro's reaction)
When we mix a strong base to a carbonyl group first the formation of an anion takes place and the reversal of the anionic charge may cause removal of the attached group, but in case of 1,2-diketone the attched group may migrate to the adjacent electron-deficient carbonyl carbon forming
Thus, benzil on reaction with a strong base forms benzilic acid (salt), reaction is known as benzilic acid rearrangement. Basically this reaction is intracannizaro reaction where formation of benzillic acid takes place.
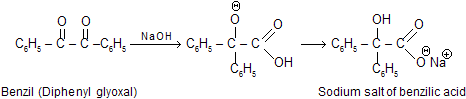
Barium hydroxide (barayta water) is more effective than sodium or potassium hydroxides due to strong basic nature. Alkoxide ions (methoxide, ethoxide, t-butoxide, etc.) in place of hydroxide ion give the corresponding esters.
Phenoxide ions are too weak for nucleophile to attack. Besides aromatic 1, 2-diketones, aliphatic and heterocyclic diketones, for example o-quinones can also undergo this type of reaction.