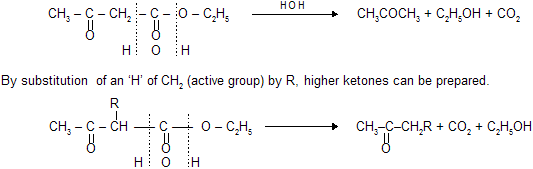Aldehydes - Ketones-1
Aldehydes and ketones
Aldehydes and ketones contains the same functional group, the carbonyl group (> C = O).
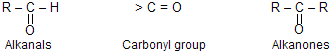
Aldehydes behave as reducing agents due to presence of reducing hydrogen atom where as ketones have no such property so aldehydes easily reacts with oxidising agent. Even HCOOH shows some properties of carbonyl compounds due to presence of – CHO group. HCOOH behaves as reducing agent while in other acids no reducing hydrogen atom, so no reaction with oxidising agent.
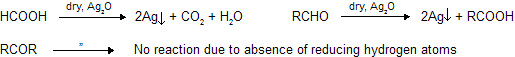
Preparation
(i) Oxidation of alcohols
(a) From PCC or pyredeniumchlorochromate. Which is pyridine, CrO3 and HCl, in equal ratio
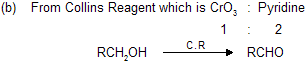
By Jones reagent (CrO3 and aq. CH3COCH3) reaction goes till acids as it is a strong oxidising agent where as by PCC and Collins reagent reaction stops at aldehyde.
(ii) Rosenmund’s reduction
Reduction of acid halide into aldehydes by Pd and BaSO4 is known as rosenmund reduction, only aldehydes can be prepared by this reaction.
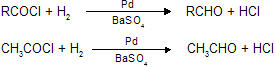
HCOCl break down into CO + HCl so the first aldehyde from rosenmund reduction is CH3CHO. Here Pd act as catalyst and BaSO4 as catalytic poison to prevent conversion of aldehyde into alcohol by further reduction.
(iii) Stephan’s Reduction
When Cyanides are partially reduced by means of stannous chloride and hydrochloric acid followed by hydrolysis yield aldehyde. Ketones can not be prepared by this process.
Here [H2SnCl4] is formed which works as powerful reducing agent.
(iv) Oxidation of alkyl halide by dimethyl sulphoxide
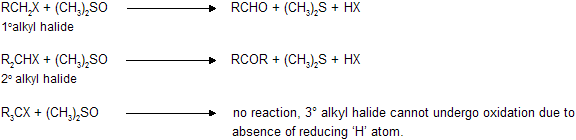
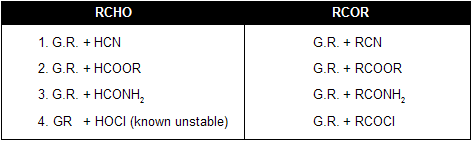
(v) From Grignard Reagent
(vi) Dry distillation of calcium salts of acids
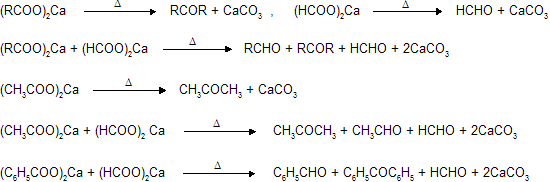
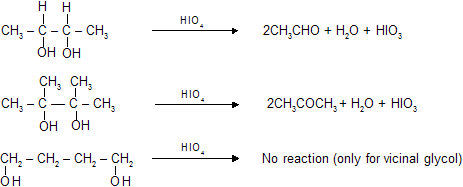
(vii) Oxidation of vicinal glycols by periodic acid
This reaction can also be done by lead tetrs acetate. We have already studied the mechanism of this reaction in last chapter.
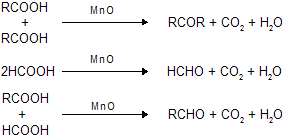
(viii) Action of MnO on acids at 300oC temprature
(ix) By Hydrolysis of Acetoacetic ester