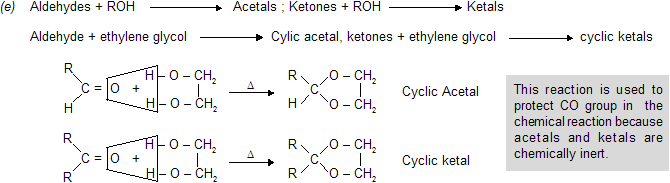
Aldehydes - Ketones-2
(x) Catalytic Dehydrogenetion by Cu at 300oC
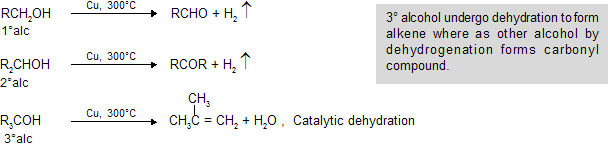
(xi) Oppenauer oxidation
This is specific method for oxidation of secondary alcohol to ketone. Secondary alcohol is heated with aluminium iso-propoxide in a ketone (usually acetone)- secondary alcohol is oxidised to ketone whereas ketone is reduced to secondary alcohol.
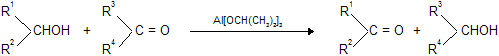
Actually this is a reversible reaction and equilibrium can be shifted in either direction by appropriate manipulation.
For example, by taking an excess of secondary alcohol, ketone can be reduced; this reduction is called Meerwein Ponndorf - Verley reduction which has been discussed later.
The uniqueness of Oppenauer oxidation lies in the fact that it selectively oxidises hydroxy group which means that if the compound besides hydroxy group has some other oxidisable functionality, the latter will remain unaffected under these conditions.
For example :
(xii) Hydrolysis of gemdihalide
Properties
(i) Nucleophilic addition reaction
Six electron system of carbocation is formed if primary attack of electrophile takes place, otherwise it is a eight electron system which is more stable when primary attack of nucleophile takes place.
So Primary attack here is of the nucleophile due to more stable intermediate oxygen anion of eight electron system. The reactivity of different carbonyl compounds towards formation of nucleophilic addition decreases in the following order :
This order arises due to the following two factors :
(i) Electronic factor : We have seen above that carbon of the carbonyl group is susceptible to the attack of nucleophile due to the presence of positive charge on it (i.e. on carbon). As the intensity of positive charge on carbon decreases, the nucleophilic attack would occur less readily. On the other hand, if intensity of positive charge increases nucleophilic attack would occur more readily. Alkyl group exerts + I effect, due to which it reduces the intensity of positive charge on carbonyl carbon, which decreases in the following order :
Intensity of positive charge on carbonyl carbon decreases. Hence the reactivity of these compounds towards nucleophilic addition also decreases in this order.
(ii) Steric factor : The change in C - C - O angle as a result of nucleophilic addition may be noted.
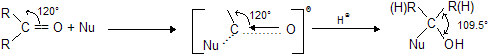
It is obvious that nucleophilic addition causes decrease in C – C – O bond angle and hence groups are pushed closer. The bigger groups oppose coming closer, hence the reactivity of carbonyl group decreases. Size of methyl group is much larger than hydrogen, hence acetaldehyde is less reactive than formaldehyde. Similarly, acetone will be less reactive than acetaldehyde.
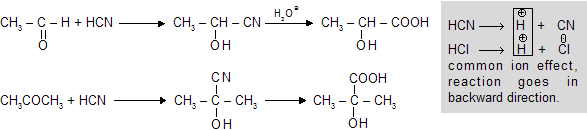
(a) Reaction with HCN
In the presence of alkali the dissociation of HCN increases because HO- of alkali trap H+ of HCN so that ionisation of HCN increases.
But in presence of acid the dissociation is suppresed due to common ion effect. Thus the reaction shifts to backward direction.
(b) Reaction with NaHSO3 (sodiumbisulphite)
This reaction is most versatile test for separating carbonyl compounds from noncarbonyl compounds, it first form white crystalline solid which when passed with dry HCl gas re-form carbonyl compound.
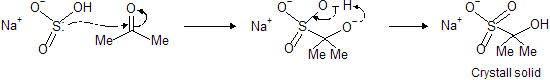
Here the carbonyl compounds are reobtained by reaction with dry HCl, so these reaction are used for separation test.
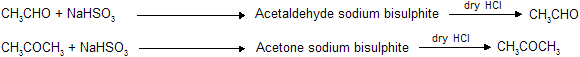
But always remember that for separation of aldehydes & ketones is done by Tollen’s reagent (ammonical AgNO3 ) i.e. [Ag(NH3)2+], where aldehydes reacts to form white metallic silver.
(c) Reaction with sodio - derivatives of 1 - alkynes
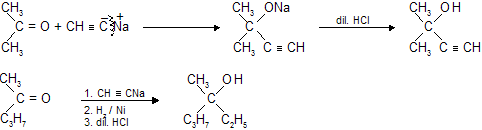
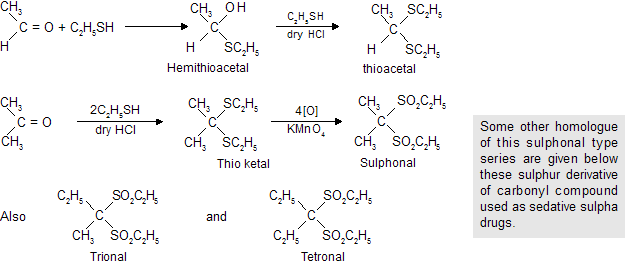
(d) Reaction with C2H5SH (Ethanethiol)