Alkadienes-1
Alkadienes and Cycloalkanes
Chapter
General formula of alkadienes is CnH2n–2
Preparation of alkadienes
(i) Dehydrogenation of butane
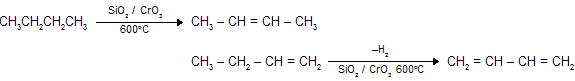
(ii) Pyrolysis of Cyclo alkenes
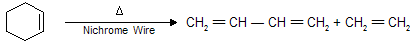
This reaction is known as Retro Diels – Alder reaction.
(iii) Dehydration of diols
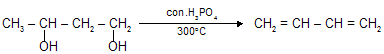
(iv) Dehydrohalogenation by alc. KOH
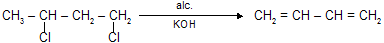
(v) From HCHO
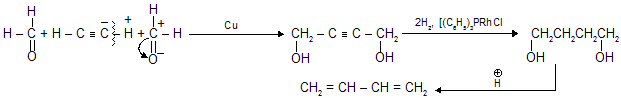
Properties of alkadienes
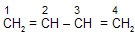
(a) In 1,3- Butadiene all carbons are in sp2 hybridisation.
(b) 1,3- Butadiene undergo resonance so that it undergo electrophilic addition reaction at both 1,2-and 1,4- position of molecule.
(c) In Butadiene C2 – C3 bond length is 1.48 Å which is less than C – C bond length of ethane while C1–C2 or C3 – C4 bond length is 1.37 Å which is slightly more than C = C of ethene.
(d) 1,3 - Butadiene undergo following type of reaction
| (i) Electrophilic addition reaction | (ii) Free radical addition reaction |
| (iii) Polymerisation | (iv) Cyclic reaction |