Alkadienes-2
[i] Electrophilic addition reaction
1,3- Butadiene undergo electrophilic addition reaction at both 1,2-and 1,4-position due to resonance.
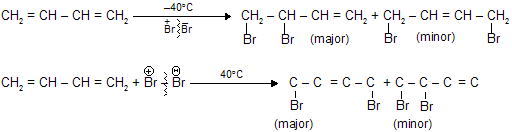
At low temperature mainly forms product of 1,2-position while at high temperature 1,4-product is the major one.
Different product 1,3-butadiene at different temperature based on concept of kinetics versus thermodynamical control.
Hydrogen halides add to 1,3-butadiene to give a mixture of two isomeric allylic halides. For the case of ionic addition of hydrogen bromide,
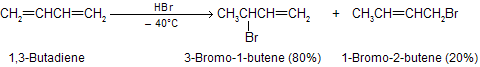
When the major product formation by addition of a proton at C-1 and bromide at C-2 in 1,3-butadiene this type of addition is called 1,2 addition, or direct addition. The minor product has its proton and bromide at C-1 and C-4, respectively, of the original diene system. This addition is called 1,4 addition, or conjugate addition. The double bond that was between C-3 and C-4 in the starting material remains there in the product from 1,2 addition but migrates to a position between C-2 and C-3 in the product from 1,4 addition.
Both the 1,2-addition and the 1,4-addition are take place from the same allylic carbocation as intermediate.
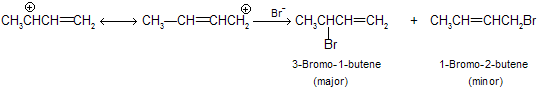
The secondary carbon atom contains more positive charge than the primary carbon, and attack by the nucleophilic bromide ion is faster there. Hence, the major product is the secondary halide.
When the major product of a reaction is the one that is formed at the fastest rate, we say that the reaction is control by kinetic control. While hydrogen bromide reacts with 1,3-butadiene at low temperature is a kinetically controlled reaction.
The ionic addition of hydrogen bromide to 1,3-butadiene is carried out at room temperature, the ratio of isomeric allylic bromides observed is different from those which are formed at low temperature. At room temperature, the 1,4-addition product gives the major part of the reaction product as thermodynamical product.
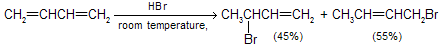
The temperature at which reaction occurs, exerts a major influence on the product composition. The 1,2- and 1,4-addition products interchange rapidly by allylic rearrangement at elevated temperature in the presence of hydrogen bromide. Heating the product mixture to 40°C in the presence of hydrogen bromide give a mixture where the ratio of 3-bromo-1-butene to 1-bromo-2-butene is 20 : 80.
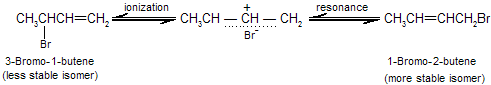
The product of 1,4 addition, 1-bromo-2-butene, contains an internal double bond and so is more stable than the product of 1,2 addition, 3-bromo-1-butene, which has a terminal double bond. To explain the concept of kinetics versus thermodynamic products, here we take addition of HCN to butenone. Two products can be formed.