Alkadienes-3
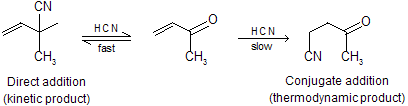
The ‘direct’ addition to the left means that cyanide ion must attack the carbonyl group directly while the ‘conjugate’ addition to the right means that it must attack the less electrophilic alkene. The second is a slower reaction but gives more stable product, point to remember.
(i) The thermodynamic product has a lower energy than the kinetic product.
(ii) Initially the reaction will go to the left, if there is enough energy for the kinetic product to get back to the starting materials, there will be enough energy for some thermodynamic product to be formed.
(iii) The kinetic product is formed reversibly ; the thermodynamic product irreversibly.
(iv) The energy needed for the thermodynamic product to get back to starting materials is very great.
(v) At low temperatures direct addition is favoured, but conjugate addition is favoured at high temperature for example 1,3-butadiene form 1-butene at low temperature by hydrogenation while at high temperature form more stable 2-butene.
We take some other example also to understand this concept, for example
| (a) | 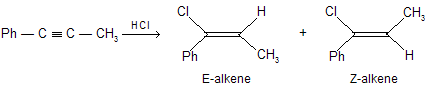 |
The two alkenes are labelled E and Z. After about 2 hours the main product is the Z-alkene. However, this is not the case in the early stages of the reaction. The graph below shows how reaction proceeds.
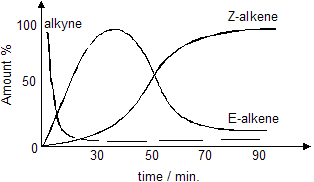
(i) When the alkyne concentration drops almost to zero (10 minutes), the only alkene that has been formed is the E-alkene.
(ii) As the time increases, the amount of E-alkene decreases as the amount of the Z-alkene increases.
(iii) Eventually, the proportions of E and Z-alkenes do not change.
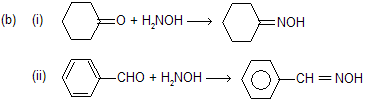
If we examine, these above reactions easily explain concepts of kinetics and thermodynamical product. After about 2 hour the main product is benzaldehydeoxime because it is more thermally stable than cyclohexanoneoxime. In early states due to more kinetics of cyclohexanone it forms major product but as the time goes and temperature also increases we prefer thermodynamical product.
When 1,3-butadiene undergo hydrogenation at room temperature by one mole of H2 it forms 2-butene as major product. Reactions of this type are said to be governed by thermodynamic control. At low temperature, addition takes place irreversibly. Isomerization is slow because insufficient thermal energy is available to permit the products to surmount the energy barrier for ionization but at higher temperatures isomerization is possible, so more stable product predominates.