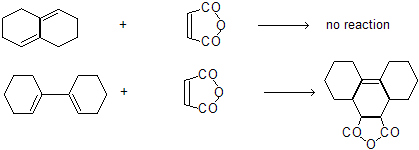
Alkadienes-5
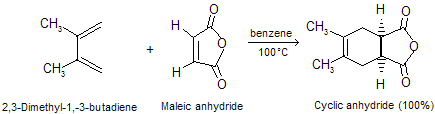
Acetylene, like ethylene, is a poor dienophile, but alkynes that bear C == O or C º N as side chain react readily with dienes. A cyclohexadiene derivative is the product.
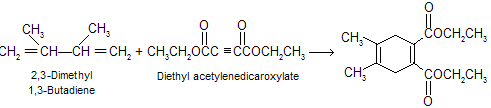
The Diels-Alder reaction is stereospecific in nature means substituents that are cis in the dienophile remain cis in the product while substituents that are trans in the dienophile remain trans in the product.
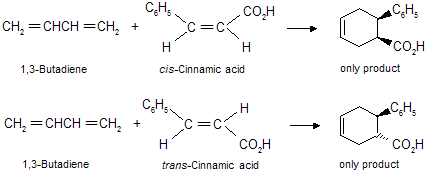
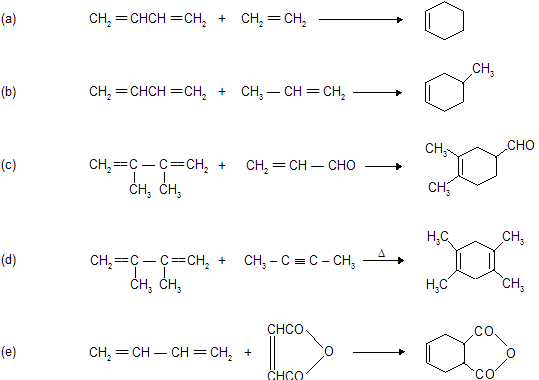
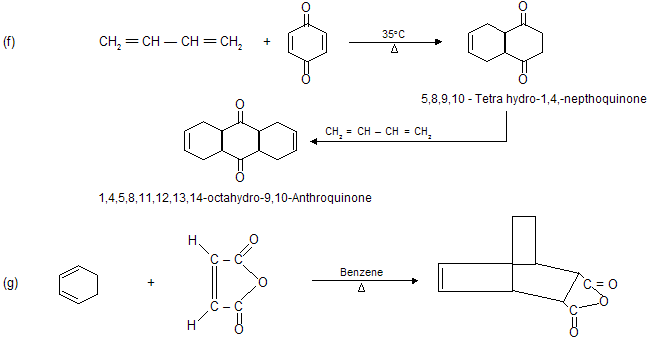
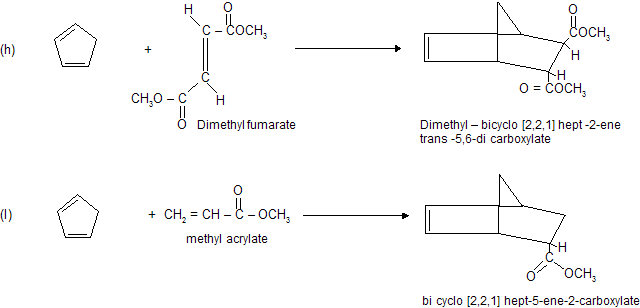
Condition for Diels alder reaction
(a) Reaction occurs between conjugated diene (enophile) and some p electron system (dienophile).
(b) Reaction always occurs at 1,4-position of conjugated diene where six member cyclic compound formation takes place.
(c) Reaction does not occur when conjugated system contains bulky alkyl group due to steric hinderance.
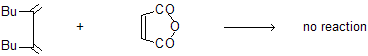
(d) Reaction occurs only in cisoid system not in transoid due to more distance.