Alkadienes-6
Diels - Alder reaction of benzyne
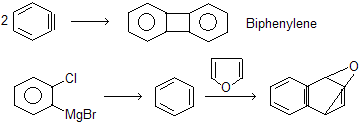
Alternative methods for generation of benzyne have made it possible to work as an intermediate in a number of synthetic applications. One such method involves treating o-Iodofluorobenzene with magnesium, normally in tetrahydrofuran as the solvent.
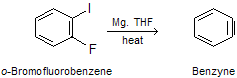
The reaction proceeds by formation of the Grignard reagent from o-Iodofluorobenzene. Since the order of reactivity of magnesium with aryl halides is ArI > ArBr > ArCl > ArF, the Grignard reagent has the structure shown below and forms benzyne by loss of the salt FMgI:
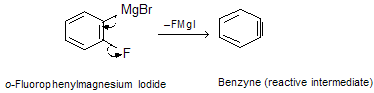
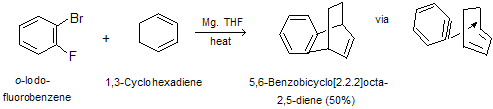
Its strained triple bond makes benzyne a relatively good dienophile, and when benzyne is generated in the presence of a conjugated diene, Diels-Alder cycloaddition occurs.
If two moles of benzyne reacts with each other they form Biphenylene.
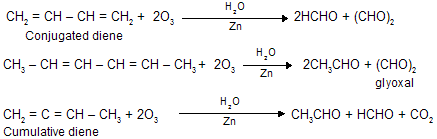
[v] Ozonolysis of alkadiene
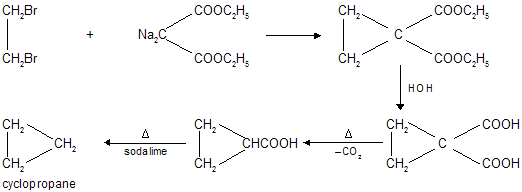
Preparation of cycloalkanes
(i) By Perkin’s Method
(ii) By pyrolysis of divalent metal salts of dicarboxylic acids (Wislicenus method)
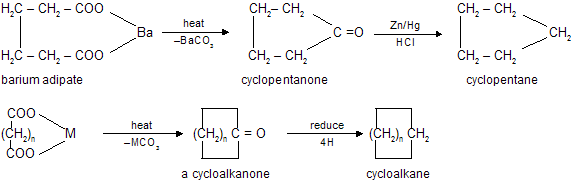
(iii) By Freund reaction
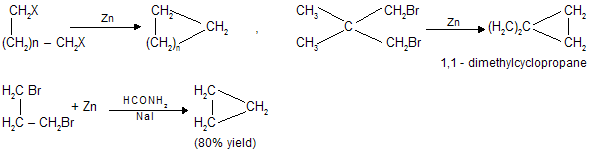
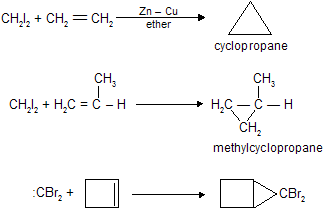
(iv) By the use of carbenes
Carbenes are unstable intermediates in which carbon has pair of unshared electrons. These are neutral species and have no formal charge, :CH2(methylene) is the simplest member of this series. On account of greater stability di halo carbenes (:CX2) are also used. Carbenes are easily prepared in solution as shown below and in the same form they are used for the reaction. This method is very useful for the preparation of small ring compounds.
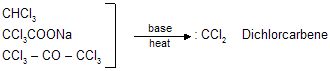
Methylene is prepared as follows

Cyclopropane and its derivatives can easily be prepared by the use of carbenes.