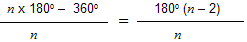Alkadienes-7
(v) Reduction of a cyclic ketone
The Wolff-Kishner carbonyl reduction is a good method for converting carbonyl group directly to methylene group. It involves heating the hydrazone of carbonyl compound in the presence of an alkali and a metal catalyst.
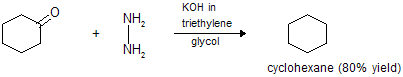
The Clemmensen’s and Wolff- Kishner carbonyl reductions complement each other, for the former is carried out in acid solution, and the latter in alkaline solution. Thus, the Clemmensen carbonyl reduction may be used for alkali-sensitive compounds where as Wolff-Kishner carbonyl reduction is the method of choice with acid sensitive compounds.
Properties of cycloalkanes
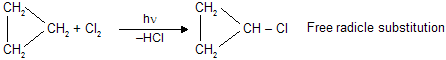
The cycloalkanes resemble the alkanes in physical properties. They are insoluble in water but soluble in many organic solvents and are lighter than water. The melting and boiling points of cycloalkanes are ten to twenty degrees higher than the corresponding alkanes (open chain molecules). But for cyclopropane and cyclobutane, cycloalkanes are relatively inert towards the action of common reagents at room temperature. These two compounds are exceptions and show a tendency to react with opening of the ring. Cyclopropane forms addition products with ring fission, as shown :

Thus cyclopropane and to a lesser extent, cyclobutane reacts by addition; all other cycloalkanes show the reactivity expected by alkanes, i.e. they react by substitution. In diffused light, cyclopropane reacts with chlorine to give substitution product.
Stability of cycloalkanes
Cyclopropane and cyclobutane resemble alkenes to a certain extent, because these compounds undergo addition reactions.
The higher cycloalkanes do not undergo addition reactions but give substitution reactions. Cyclopropane and its derivatives are most reactive and least stable where as, cyclobutane is less reactive and cyclopentane is still less reactive more stable.
Adolf Van Baeyer, a German chemist, in 1885, proposed a theory to explain the relative stability of cycloalkanes. The theory is based on the fundamental concept of regular tetrahedral structure as given by Vant Hoff and Le Bel. According to them “ the four valencies of carbon atom are directed towards the four corners of a regular tetrahedron with carbon atom at the centre making an angle of 109o28’ between any pair of such valencies.”
According to Baeyer’s strain theory the valence angles can be altered from the normal value of 109o28’ and when this alternation is done, an internal strain is setup in the molecule. The greater the deviation from the normal angle, greater is the strain and consequently lesser is the stability of the molecule. The main assumption in the theory is that all the carbon atoms forming the ring must lie in the same plane. Thus the degree of stability of ring is inversely proportional to the amount of deviation from the normal angle of 109o28’. Bayer proposed that this deviation could be calculated as shown :
 = ½(109°28’– bond angle) where all the carbon atoms in the ring are in the same plane. Suppose there are n carbon atoms in the ring, then the bond angle of ring would be
= ½(109°28’– bond angle) where all the carbon atoms in the ring are in the same plane. Suppose there are n carbon atoms in the ring, then the bond angle of ring would be