Alkadienes-8
Most of the compounds tends to convert in stable ring like we know that cyclopropane is maximum strain ring so that it want to convert in cyclopentane and cyclohexane derivatives in different chemical reaction for example.
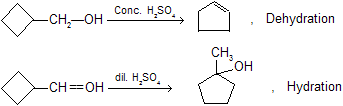
In all above example due to formation of intermediate carbocation, expansion of ring can takes place.
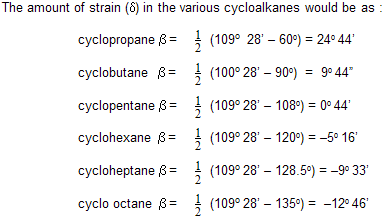
It can be seen from the above data that the lowest value of the devitation is found in five and six-membered rings and so cyclopentane and cyclohexane are the most stable systems.
The negative value of d indicates that the bonds are bent outwards. The Baeyer’s strain theory could easily explain the greater reactivity of cyclopropane and cyclobutane and the stability of cyclopentane and cyclohexane rings. The strain theory agrees reasonably with properties of alicyclics containing six or less number of carbon atoms it is now clear that why only the 1,4 and 1,5 dicarboxylic acids form cyclic anhydrides whereas 1,6 and 1,7 form cyclic ketones etc.
Degree of Unsaturation (DU)
The number of pairs of H atom a molecular formula lacks to be an alkane is called the degree or element of unsaturation (DU). The number of elements of unsaturation.
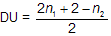
Where n1 is number of carbon atom while n2 is number of hydrogen atom.
# 1 degree of unsaturation C == C bond or one ring
# 2 degree of unsaturation means C == C, or one C == C or two ring or one ring and one C == C bond.
# Halogen is like H, O is not counted in oxygen containing compounds and one H is counted less in N containing compounds.
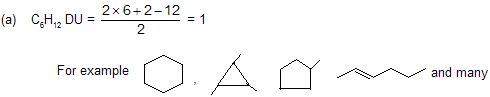
b) C8H12 elements of unsaturation DU = 3

You can also make many other arrangements
# C3H3Cl3 (It is like C3H6) = 1° unsaturation
# C3H4O (It is like C3H4) = 2° unsaturation
# C4H5N (It is like C4H4) = 3° unsaturation
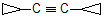 this molecule contains four degree of unsaturation because cyclopropyl ring is there which undergo addition reaction.
this molecule contains four degree of unsaturation because cyclopropyl ring is there which undergo addition reaction.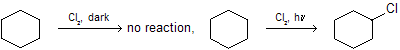
In above example cyclohexane is stable ring, so it cannot react in dark but in the presence of sunlight free radical substitution reaction occurs.