Alkanes-Paraffins-3
(v) Reduction of alkyl halides
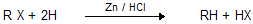
reduction can also be done by a. LiAlH4 b. Na/C2H5OH c. Zn – Cu Couple
Zn – Cu couple is Zn wetted by Cu. It is prepared by putting CuSO4 on Zn granules present in flask which leads to Cu deposition on them.
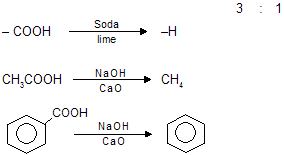
(vi) Wurtz Reaction
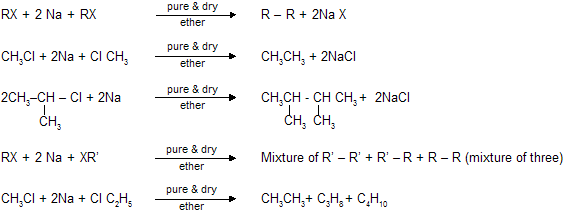
Two following mechanism have been suggested for wurtz reaction:
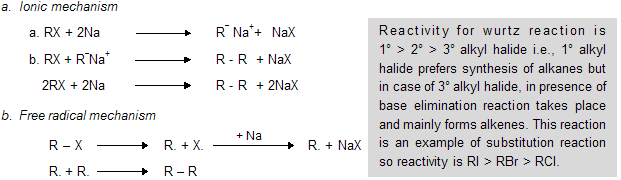
If we take one alkyl and one aryl group, this is known as wurtz fittig reaction (practically possible)
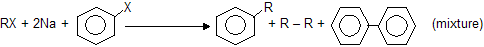
(vii) Sabatier Senderon’s Reduction (Hydrogenation of alkyne or alkenes)
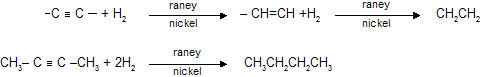
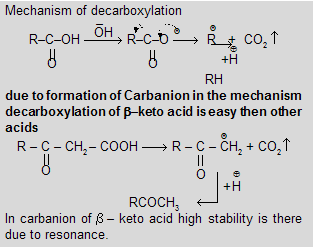
(viii) Decarboxylation by Sodalime (NaOH + CaO)
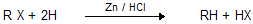
reduction can also be done by a. LiAlH4 b. Na/C2H5OH c. Zn – Cu Couple
Zn – Cu couple is Zn wetted by Cu. It is prepared by putting CuSO4 on Zn granules present in flask which leads to Cu deposition on them.
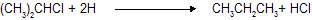 |  |
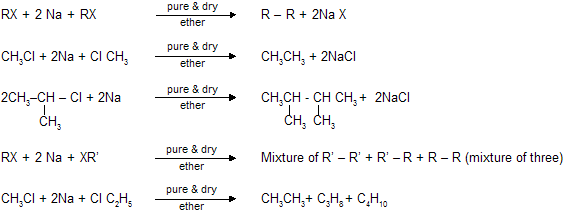
Two following mechanism have been suggested for wurtz reaction:
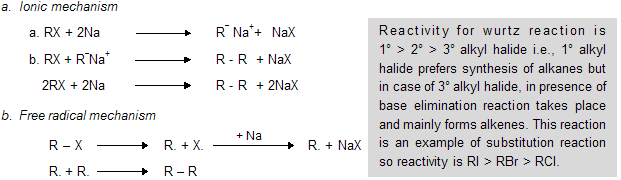
If we take one alkyl and one aryl group, this is known as wurtz fittig reaction (practically possible)
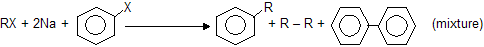
(vii) Sabatier Senderon’s Reduction (Hydrogenation of alkyne or alkenes)
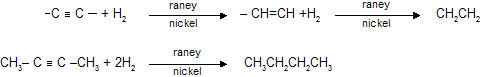
(viii) Decarboxylation by Sodalime (NaOH + CaO)
 |  |