Alkanes-Paraffins-4
(ix) Hydrolysis of metal carbides

Al & Be both gives same alkane due to diagonal relationship between them. 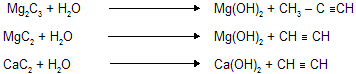
|  |
Wolff–Kischner reduction involves the conversion of carbonyl groups of carbonyl compounds in to methylene groups by heating their hydrozones or semicarbazones in the presence of strong base such as C2H5ONa or NaOH or KOH.
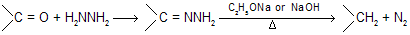
Earlier method of heating the hydrozones of carbonyl compounds with C2H5ONa at 180°C in an autoclave has since been modified. In the modified procedure, hydrazine hydrate and the carbonyl compound are heated with KOH or NaOH in ethylene glycol for several hours. (Hung minlon reaction) The water formed escapes and the temperature rises to 200°C when the hydrazone decomposes with the formation of hydrocarbon with evolution of nitrogen due to more boiling point of solvent all water easily separate out.
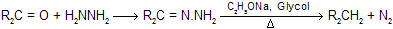
In a further modification, the reduction can be carried out at room temperature by using dimethyl sulphoxide as solvent and potassium tertiary butoxide as base. The yield is very good for example.
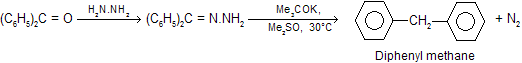
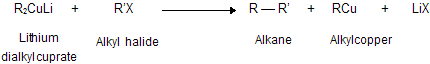
(xi) Corey house synthesis
The most useful organocopper reagents are the lithium dialkylcuprates, which is generated when a copper (I) halide reacts with two equivalents of an alkyllithium in ether as a solvent like tetrahydrofuran.
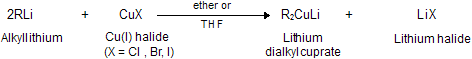
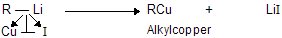
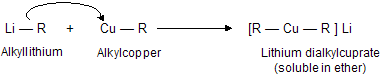
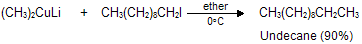
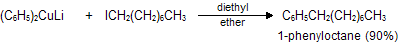
As we have seen methane, ethane, propane, and butane are gases at room temperature. The unbranched alkanes from pentane (C5H12) to heptedecane (C17H36 ) are liquids, while higher homologous are solids. The boiling points of unbranched alkanes increase as the number of carbon atoms in the chain increases. The boiling points for 2-methyl-branched alkanes are lower than those of the unbranched isomer. By exploring the molecular level the reasons for the increase in boiling point with the number of carbons and the reasons for the difference in boiling point between branched and unbranched alkanes, we can begin to develop some insights into the relationship between structure and properties.
A substance exists as a liquid rather than a gas because there are cohesive forces between molecules (intermolecular attractive forces ) that are greater in the liquid state than in the gas phase. Attractive forces between neutral species (atoms or molecules, but not ions) are referred to as Vander Waals forces and they are
1. London forces
2. dipole - dipole
3. ion - dipole
4. hydrogenbonding.
The intermolecular interactions, which are observed in nonpolar molecules, are known as London forces. The electrons are in continuous movement and induce temporary polarization in one molecule, which in turn induces polarization in an opposite direction in adjacent molecule. This momentary induction of dipole results in attraction between otherwise nonpolar molecules (induced dipole interaction). The London forces are very weak intermolecular forces. The attraction among nonpolar molecules of alkanes is attributed to London forces.