Alkanes-Paraffins-5

Melting point. The temperature at which a solid gets converted to a liquid is defined as the melting point of that solid. In solid state, the particles are arranged in highly ordered manner (regular and symmetrical). On heating, the particles acquire thermal energy and move to a random arrangement as in liquid. The temperature at which thermal energy of particle overcomes the forces of attraction holding them in an orderly arrangement is known as the melting point.
In general, ionic compounds have much higher melting points compared to covalent compounds. In ionic compounds, the strong electrostatic forces of attraction hold the oppositely charged ions in a crystal lattice. To overcome the strong forces, we have to break the ionic bonds between oppositely charged ions and for that a very high temperature is required.
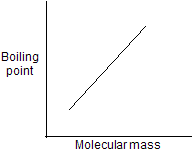
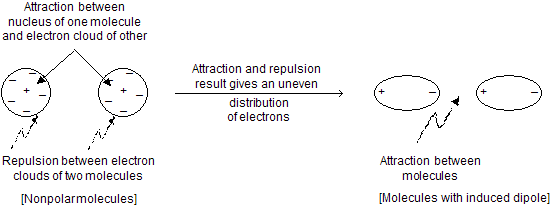
In covalent compound, the molecules are held together in crystal through weak van der Waal forces of attraction (dipole-dipole or induced dipole interactions). To overcome these forces, relatively low temperature is required. Unlike ionic compounds, no bonds are broken in covalent compounds rather weakly held molecules are to be separated from each other. In general, the melting point of organic compounds increases with an increase in molecular mass.
Melting point depends upon
| 1. Molecular symmetry | 2. Molecular mass |
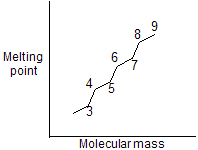
For example in normal pentane and neopentane, higher melting point of neopentane due to more symmetry and compact structure. Other example is trans form which always contains higher melting point than cis form due to more stability and symmetry.
In molecules of alkanes melting point follows saw tooth rule means odd number alkanes does not get much higher melting point than even number due to less symmetry and more repulsion of carbon edges. So graph comes in zigzag manner.
Boiling point depends upon
| 1. Molecular mass | 2. Polarity of molecule |
Cis isomer are more polar so higher boiling point than trans isomers.