Alkanes-Paraffins-8
(iii) Nitration
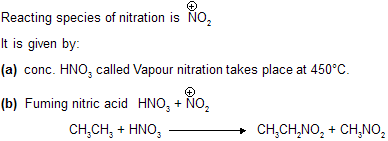
Here mixture is obtained due to cleavage of C–C and C–H Bond.
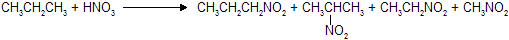
Mixture of nitroalkanes are formed in different percentages where product amount based on intermediate stability.
(CH3)2CHNO2 is obtained in highest proportion on nitration of propane while nitroethane and nitromethane like compounds in minimum amount.
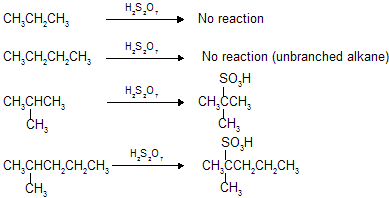
(iv) Sulphonation
This process is done by con. H2SO4 or fuming H2SO4 (olium : H2S2O7) which contains SO3 as active species, but in this process by the mechanistic rule –H atom is replaced by –SO3H group.
We already know that alkanes are less reactive so lower alkanes do not undergo sulphonation however only higher branched alkanes undergo sulphonation with fuming H2SO4 (oleum). Alkanes are very less reactive so require reagent with high reactivity at high temperature so fuming H2SO4 (oleum) which contains dissolved SO3 works as suitable sulphonating agent.
Q: Which of the following does not undergo sulphonation ?
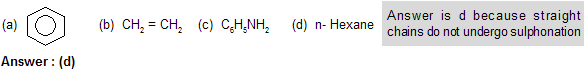
(v) Combustion
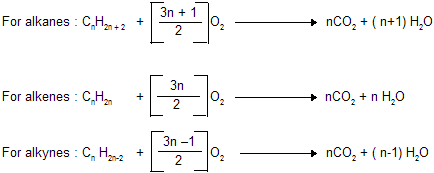
Q: How many litres of air is needed for complete combustion of 8 lit. of CH º CH ? |  |
| Answer = 100 litres |