Alkanes-Paraffins-9
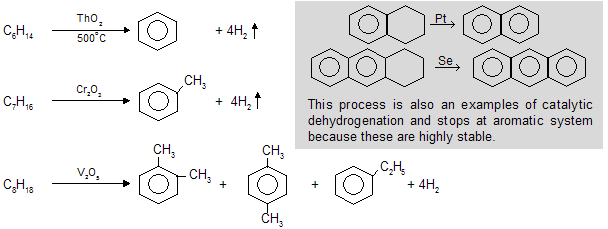
(vi) Catalytic dehydrogenation / Hydroforming / Cyclization / Aromatisation
When alkanes with at least six carbon atoms are heated in the presence of oxides of d-block elements e.g. Mn,Co, Th, V, Cr (good catalyst) to form six carbocyclic aromatic compounds with higher stability.
(vii) Pyrolysis: Cracking
Decomposition of a compound by the action of heat alone is known as Pyrolysis. This word is taken from the Greek pyre = fire, and lysis = loosing and hence to chemists it means “cleavage by heat”, compare hydro-lysis “cleavage by water”.The pyrolysis of alkanes, particularly when petroleum is concerned, is known as cracking. In thermal cracking alkanes are simply passed through a chamber heated to high temperature. Large alkanes are converted into smaller alkanes, alkenes, and some hydrogen. This process yields predominantly ethylene (C2H4 ) together with other small molecules. In a modification called steam cracking, the hydrocarbon is diluted with steam, heated for a fraction of a second to 700 - 900oC, and rapidly cooled. Steam cracking is of great importance in the production of hydrocarbons as chemicals, including ehtylene, propylene, butadiene, isoprene and cyclopentadiene. Another source of smaller hydrocarbons is hydrocracking, carried out in the presence of catalyst and hydrogen, at high preasure and at much lower temperatures (250 - 450oC).
The low-molecular weight alkenes obtained from these cracking processes can be separated and purified and are the most important raw materials for the large scale synthesis of aliphatic compounds.
Most cracking, however, are directed toward the production of fuels, not chemicals, and for this catalytic cracking is the major process. Higher boiling petroleum fractions (typically, gas oil) are brought into contact with a finely divided silica-alumina catalyst at 450 - 550oC and under slight pressure. Catalytic cracking not only increases the yield of gasoline by breaking large molecules into smaller ones, but also improves the quality of the gasoline; this process involves carbocations and yields alkanes and alkenes with the highly branched structures desirable in gasoline.
Through the process of alkylation some of the smaller alkanes and alkenes are converted into high-octane synthetic fuels.
Finally, by the process of catalytic reforming enormous quantities of the aliphatic hydrocarbons of petroleum are converted into aromatic hydrocarbons which are used not only as superior fuels but as the starting materials in the synthesis of most aromatic compounds.
Pyrolysis follows free radical mechanism
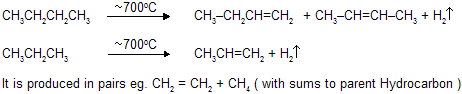
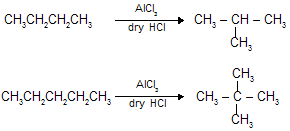
(viii) Isomerisation :
When straight chain alkanes are heated in the presence of AlCl3 and dry HCl gas they are converted into more symetrical stable branched chain alkane.
USES OF ALKANES
