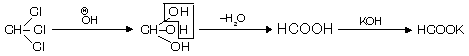Alkyl-Halides-2
The trihalomethyl ketone (RCOCX3) so formed when undergoes nucleophilic addition reaction of hydroxide ion to its carbonyl group, and finally its dissociation by cleavage, of the bond to the CX3 group.
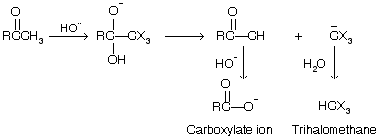
The three –I effect halogen group stabilize the negative charge of the trihalomethide ion (C–X3) converting it to weak conjugate acid and permitting it to act as a good leaving group in the carbon bond-cleavage step or 2-alkanone.
This reaction is also used for the preparation of carboxylic acid from methyl ketones.
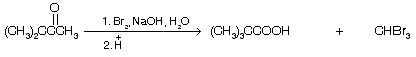
(ii) Haloform test from bleaching powder (CaOCl2)
It is also used as reagent for haloform test due to the presence of OQCl which is a strong base.
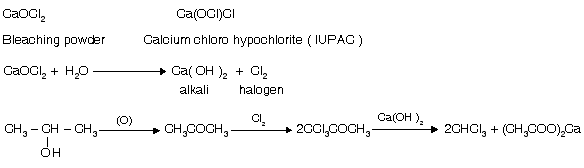
(iii) From chloral hydrate
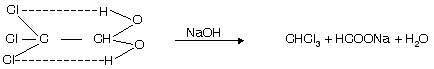
It shows intramolecular H-bonding and is thus stable. This method is used to prepare fresh and pure chloroform.
(iv) Reduction of CCl4
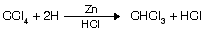
Properties of chloroform
(i) Oxidation
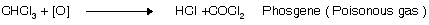
Storage of CHl3
a. It is stored in amber coloured bottles up to the brim in dark.
b. Small amount of ethyl alcohol is added which converts poisonous phosgene into nontoxic compound, diethyl carbonate.
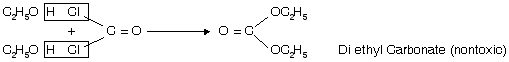
(ii) Testing of Chloroform
a. Pure chloroform does not react with AgNO3 due to absence of Cl¯ ion
b. Impure chloroform or oxidised chloroform gives white ppt with AgNO3 due to presence of HCl which contains Cl- ion.

(iii) Reaction with HNO3
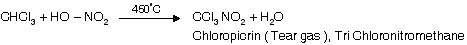
(iv) Reaction with acetone
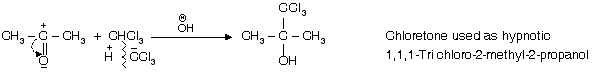
(v) Reaction with Benzene
Substitution of halogen by phenyl group occurs in the presence of Lewis acids
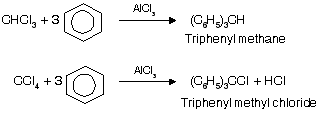
In case of CCl4 only 3’Cl’ atoms are replaced by phenylgroup, while 4th ‘Cl’ atom donot undergo replacement by phenyl group due to high stable intermediate triphenyl methyl carbocation.
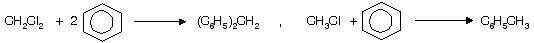
(vi) Reaction with aq. KOH
Nucleophilic substitution reaction occurs where ‘Cl’ is substituted by –OH group.