Alkyl-Halides-3
(vii) Hoffmann’s Carbylamine Reaction
| When all primary amines reaction with chloroform and alc. KOH theyform isocyanides with bad smell. It is the most versatile test for all primary amines. In this a-elimination occurs. | 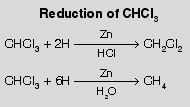 |
 |
Mechanism
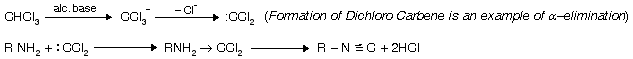
Separation Tests of some Pairs ?
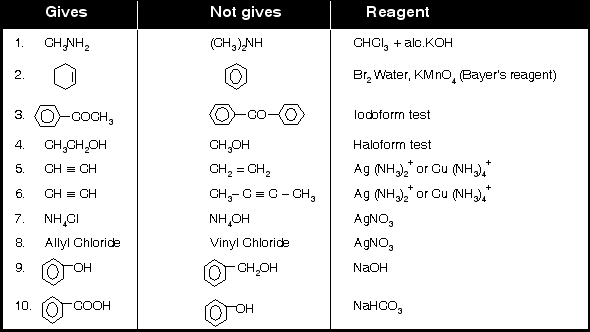
(viii) Reimer Tiemann’s Reaction
Process of formylation of phenols with chloroform in alkaline solution is known as Reimer–Tiemann reaction.
A mixture of ortho-and para-isomers is obtained in which the ortho isomer predominates due to more thermodynamical stability. If one of the ortho positions is occupied the para-isomer is the major product.
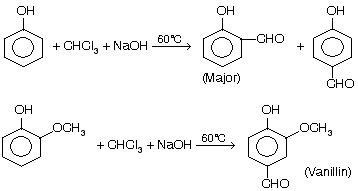
The reaction is carried out by refluxing an alkaline solution of phenol and chloroform at 60°C for sometime (1/2 h). Excess chloroform is distilled off, the mixture acidified with sulphuric acid and steam distilled. Unreacted phenol and the ortho-isomer distil over leaving behind the para isomer. The two isomers are further purified by sodium bisulphite.
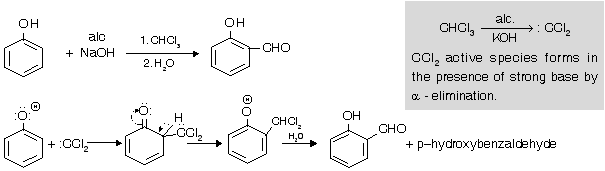
Applications
The reaction mainly used for introducing aldehyde or carboxyl group in phenols.
(i) Preparation of vanillin
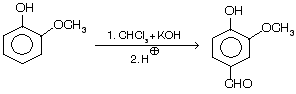
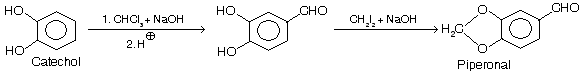
(ii) Preparation of piperonal
(iii) Formylation of heterocyclic compounds
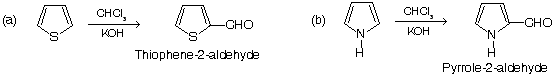
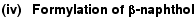
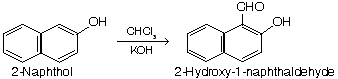
(v) Preparation of acid (salysylic acid)
When carbon tetrachloride in place of chloroform is used, a carboxyl group is introduced.
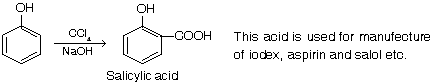
(vi) Abnormal Reimer–Tiemann reaction (where ring expansion takes place due to electrocyclic rearrangement)
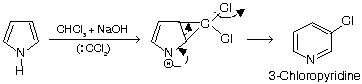
1. Pyrrole undergoes normal and abnormal RTR
Normal reaction (pyrrole is ortho & para activating compound).
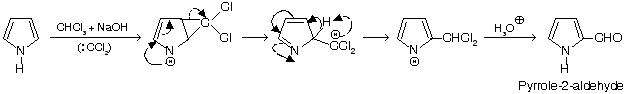
Abnormal reaction (ring expansion)