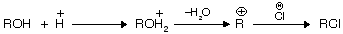Alkyl-Halides-4
Pyrene CCl4
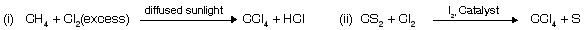
CCl4 does not react with H2O while SiCl4 reacts due to presence of vacant d–orbital in Si atom used by water molecule as nucleophile. CCl4 at high temperature reacts with H2O where vacant orbital of carbon at high temperature as used by water to form phosgene.
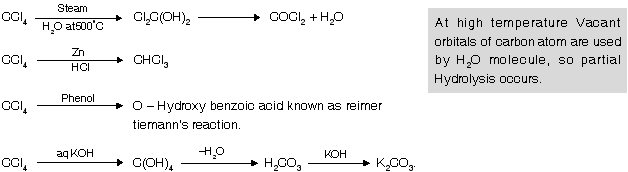
Freons : Poly chloro fluoro alkanes are known as freons. They are colourless, odourless, non toxic, non inflammable liquids with very less chemical reactivity & high stability. They are used in refrigerators and air conditioners for cooling purpose and as propellents in rockets and jets.
Ozone hole
Chloroflorocarbons known as freons, commercially used for refrigeration purpose they are highly volatile and stable in nature (life time is more than 100 years) due to this they easily move up into the higher zone of atmosphere (stratosphere), and reacts with ozone causing hole resulting in to as ozone depletion.
Reaction follows free radical mechanism
(i) Chain initiation step
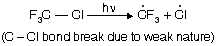
(ii) Chain propagation step
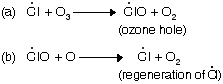
The damage of ozone layer in stratosphere clear the path for harmful ultraviolet radiation to come on earth’s surface.
Now a days hydroflorocarbons (HFC’S) are used as substitutes for CFC’S because CFC’S are involved in depletion of ozone layer. Ozone has a tendency to absorb uv rays and works as natural shield from harmful rays.
DIHALOGEN DERIVATIVES

Preparation

Properties
Ethyledene chloride and Ethylene chloride gives reactions with following reagents in almost same pattern by nucleophilic substitution reaction, for example

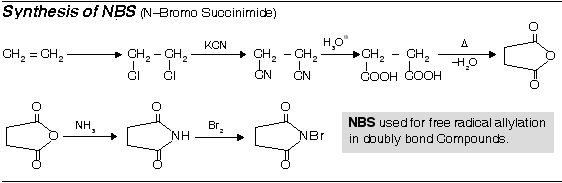
Preparation of Mono halogen derivatives (RX)
(i) From alcohol

It is the (Darzon’s) best method for preparation of halide from alcohol because pyridine forms a complex with HCl. SO2 is liberated, thus pure RX is obtained. Pyridine behaves as a weak base due to the presence of lone pair of electrons.
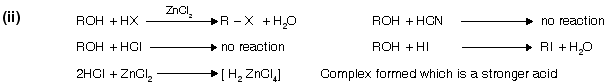
alcohols do not react directly with HCl but react in presence of ZnCl2 because in its presence they form a complex which is stronger acid than HCl itself.