Alkyl-Halides-5
Order of reactivity of halogen acid HI > HBr > HCl, because acid which give quickly. Proton is maximum reactive while order of reactivity of alcohol is 3o > 2o >1o > CH3OH due to intermediate carbocation formation where more the stability of intermediate higher the reactivity.
(iv) From 1° amines with NOCl or Tildens reagent (nitrosyl chloride)
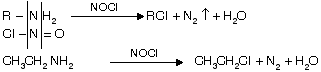
(v) From alkenes
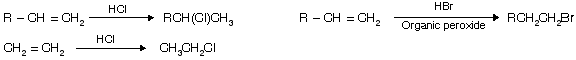
(vi) Halogenation of alkanes

This reaction involves decarboxylation and Bromination both simultaneously. This reaction is used for bromides only. It is used to prepare lower homologues (to step down) in alkyl halide series.
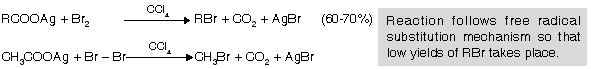
(viii) Substitution of one halogen by another (Halogen exchange)
In the reaction described in earlier part of this chapter, we have already taken examples of nucleophilic substitutions involving halide leaving groups. Halide ions may also works as nucleophiles. In a reaction known as halogen exchange, one halogen displaces another from an alkyl halide.
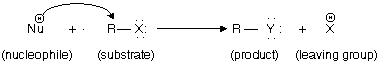
Since the halide which undergo displacement is also a nucleophile, an equilibrium is established. Synthetic chemistry application is how to shift the position of equilibrium so as to make this reaction an effective and spontaneous one for the preparation of alkyl fluorides and alkyl iodides.
In the preparation of alkyl fluorides, an alkyl chloride, bromide, or iodide is heated with potassium fluoride in a high boiling alcohol solvent such as propylene glycol.
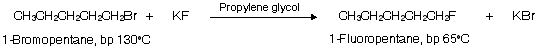
Alkyl fluorides have the lowest boiling points (Lowest mass) of all the alkyl halides and are removed from the reaction mixture by distillation as they are formed. According to Le Chatelier’s principle, the system responds by forming more alkyl fluoride at the expense of the original alkyl halide. Even if the alkyl fluoride were not removed by distillation, it would predominate at equilibrium because the reaction favours spontaneity of the stronger C — F bond in place of weaker C –– I, C –– Br, or C –– Cl bonds of low dissociation energy. Since synthetic method for these compounds, this reaction is known as Swart’s reaction.
Alkyl iodides may be prepared from alkyl chlorides and bromides by treatment with sodium iodide in acetone as the solvent, known as (Finkelstein) connent reaction.
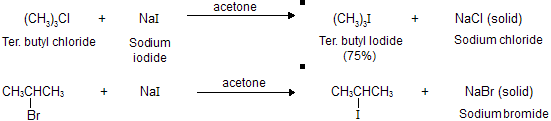
Le Chatelier’s principle under go application in this reaction. Sodium iodide is soluble in acetone due to more covalent nature but sodium bromide and sodium chloride are not. In these reactions, sodium chloride and sodium bromide precipitate from the reaction mixture, causing the position of equilibrium to shift in forward direction (activemass is unity for solid) so as to favour formation of the alkyl iodides.
Physical Properties
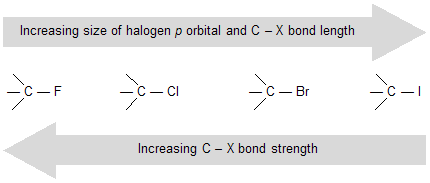
(a) Bond strength: In haloalkanes bond strength of carbon–halogen bond decrease with an increase in bond length, as one moves from fluorine to iodine. This is attributed to the size of p orbital, which increases from fluorine to iodine and thus makes the sp3 -p overlap less effective.
(b) Solubility and state of matter: Haloalkanes are insoluble in water but soluble in organic solvents. Lower molecular mass haloalkanes are gases at room temperature while higher molecular mass haloalkanes are liquid at room temperature.
(c) Boiling point: Boiling point of haloalkanes is higher compared to corresponding alkanes due to dipole–dipole interaction. With an increase in molecular mass, there is an increase in boiling point. For the same alkyl group the boiling point increases from fluoroalkane (R–F) to iodoalkane (R–I). Iodine has a larger surface area and outer electrons are loosely bound. This makes iodine a highly polarizable atom. A polarizable atom has increased London forces of attraction (section 1.7), which causes an increase in boiling point. The branched chain haloalkanes follow a similar increasing order of boiling points.
(d) Density: The densities of haloalkanes increase with atomic mass of the halogen and decrease with increasing size of the alkyl group. In monohaloalkanes, iodomethane (CH3I) has the maximum density.
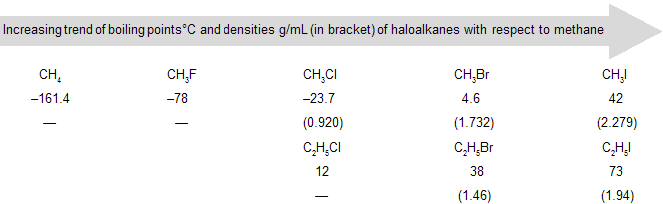
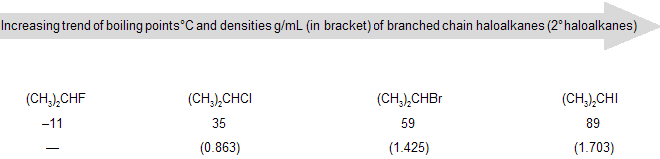
(a) Fluoro and chloroalkanes are less dense than water whereas bromo and iodoalkanes are denser than water.
(b) Monofluroalkanes are unstable and on heating, H–F is eliminated to produce alkene.
(c) Bromo and iodoalkanes are generally photosensitive and are stored in brown opaque bottles. Otherwise, they liberate free bromine and iodine respectively.
Properties of mono halogen derivatives
Mono halogen derivatives follows nucleophilic substitution reactions which are mainly of SN1, SN2 type. Reaction follows which type of mechanism is mainly decided by halide quality and medium (solvent). Detail study of mechanism is given in the next chapter of the book.