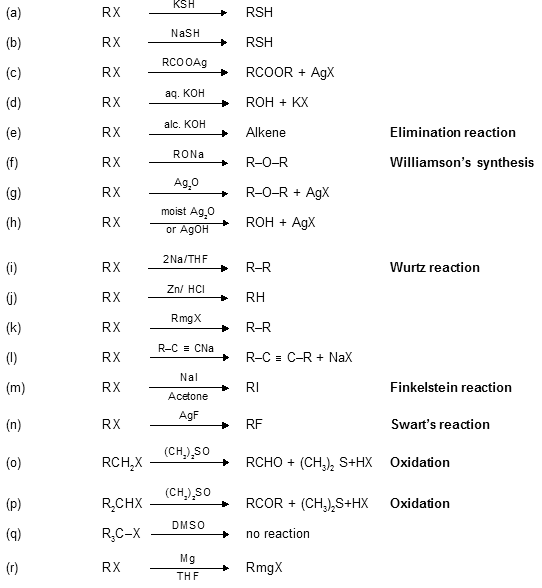
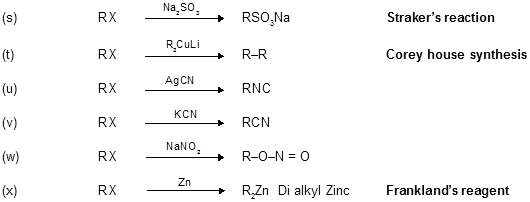
Alkyl-Halides-6
(i) Synthesis of cyanide and Isocyanide

With KCN, halides mainly form cyanides because in KCN molecule ionic bonding occurs which produces CN- with ambident nature where two sites of attachment are there as negative charge and lone pair of electrons. Attachment prefers at carbon site because negative charge is more active than pure lone pair. Reaction with AgCN (covalent bonding) produces isoaganides as major product.

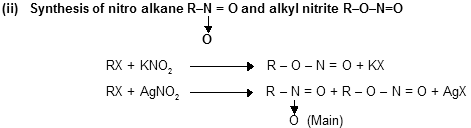
NO2 also works as ambident nucleophile because it has two sites for attachment one is nitrogen lone pair while other is negative charge on oxygen atom. There is ionic bonding in KNO2 molecule so it produces exclusively alkyl nitrite while with AgNO2 (covalent) attachment prefer from nitrogen site because lone pair of nitrogen is more reactive than lone pair of oxygen.

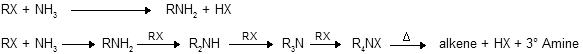
When primary halides reacts with ammonia to form primary amines as major product but when excess of alkyl halides are taken than exhaustive alkylation occurs, where synthesis of quaterary ammonium salts takes place. By heating they undergo b-elimination through Hoffmann’s orientation forms less substituted alkenes as major product.
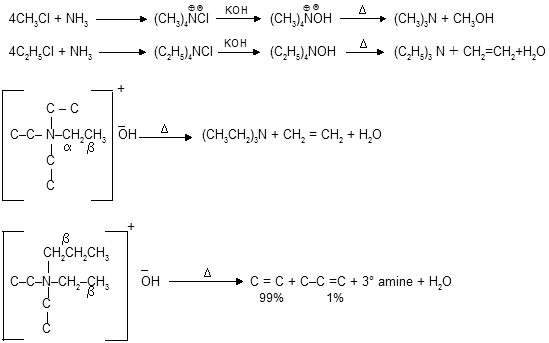
In above cases alkene formation takes place by ethyl group & not by propyl group because b–carbon of propyl is 2° in nature & abstraction of proton order in case of Hoffmann’s b–eliminations is 1°>2°>3° carbon atom otherwise –CH2NO2 > –CH2Cl > 1°. If any –I effect group is present on b carbon it increases the reactivity for elimination from that site.
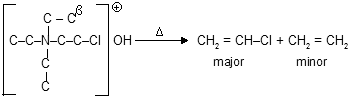
Other chemical reaction mainly follows E-1 or E-2 reaction for elimination (dehydration and dehydrohalogenation) where sytzeff’s rule for stabilization occurs for example.
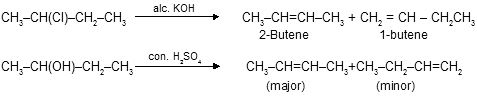
(iv) Some other synthetic methods from alkyl halide