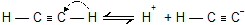Alkynes-1
General formula of alkynes is CnH2n–2
They are more acidic then alkene and alkane because they have sp hybridised carbon atom which is more elctronegative.
Preparation
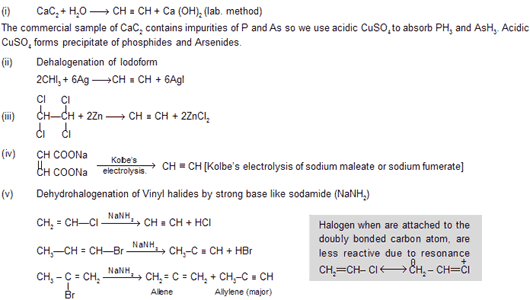
Properties
Alkynes resemble alkanes and alkenes in their physical properties. They share with those other hydrocarbons the properties of low density and low water-solubility. They are nonpolar and dissolve readily in typical organic solvents such as alkanes, and diethyl ether. Alkynes generally have slightly higher boiling points than the corresponding alkanes and alkenes due to more polar character. Normally they are colourless and odourless gas - acetylene is also colourless and odourless but it contains garlic odour due to impurities of PH3 & AsH3.
(i) Acidity of acetylene and terminal alkynes
The C––H bonds of hydrocarbons show little tendency to ionize, and alkynes are very weak acids. The ionization constant (Ka) for methane, for example, is too small to be measured directly, but is about 10-60 (pKa 60).
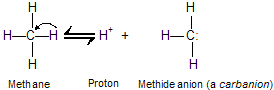
Since it is derived from a very weak acid, a carbanion such as CH3 is an exceptionally strong base. In general, the ability of an atom to bear a negative charge is related to its electronegativity. Both the electronegativity of an atom X and the acidity of H––X increase across a row in the periodic table.
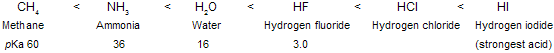
In above HI is strongest acid so, the basicity of the conjugate base : X–- decreases across this series.
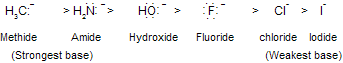
As the electron-attracting power of the negatively charged atom increases, the anion becomes less basic. Alkynes are more acidic than alkenes, and alkenes are more acidic than alkanes.
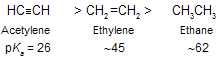
This order of acidity by examining how strongly each anion holds the unshared electron pair. The ionization of acetylene yields an anion in which the unshared electron pair occupies sp hybridisation.