Alkynes-2
Ionization of ethylene gives an anion in which the unshared electron pair occupies sp2 hybridisation.
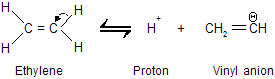
The equilibrium constant for ionization of acetylene is greater than that of ethylene because an electron pair in an orbital with 50 percent s character (sp) is more strongly bound than an electron pair in an orbital with 33% s character (sp2). Terminal alkynes (R – C == CH) are similar to acetylene in their acidity where all 1-alkyne contains acidic hydrogen atom.
While acetylene and terminal alkynes are far stronger acids than other hydrocarbons, it must be remembered that they are, nevertheless, very weak acids–– much weaker than water and alcohols. For example, hydroxide ion is too weak a base to convert acetylene to its anion in good amounts. The position of the equilibrium described by the following equation lies overwhelmingly to the left:
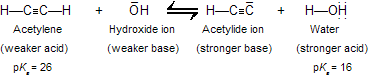
Because acetylene is a far weaker acid than water and alcohols, these substances are not suitable solvents for reactions involving acetylide ions. Acetylide is instantly converted to acetylene by proton transfer from compounds that contain hydroxyl groups.
Amide ion is a much stronger base than acetylide ion and converts acetylene to its conjugate base quantitatively.
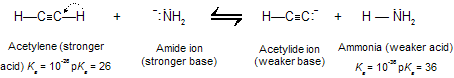
Solutions of sodium acetylide (HC=CNa) may be prepared by adding sodium amide (NaNH2) to acetylene in liquid ammonia as the solvent. Terminal alkynes react similarly to give species of the type RC=CNa.
Anions of acetylene and terminal alkynes are nucleophilic and react with methyl and primary alkyl halides to form carbon-carbon bonds by nucleophilic substitution. Some useful applications of this reaction will be discussed in the following section.
Alkynes are acidic in nature because as the ‘s’ character increases electrons are drawn closer to the nucleus.Electronegativity order sp > sp2 > sp3 so that following reactions express the acidic nature of 1 - alkynes.
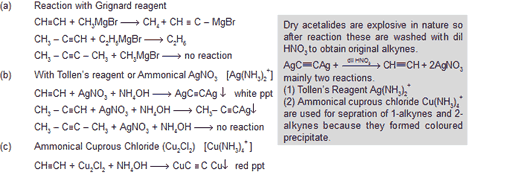
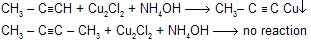
(d) With Ammonical I2
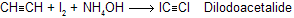
(e) With Na / liquid NH3
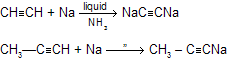
(f) With Cl2 / FeCl3
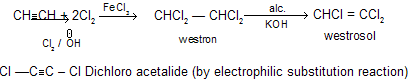
Westron and westrosol are used as solvent in dry cleaning industries.