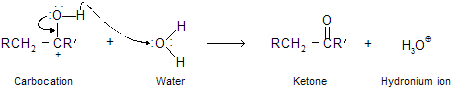Alkynes-3
(ii) Hydrogenation of alkynes
The hydrogenation of alkynes are similar to those employed for alkenes. When reaction occurs in presence of finely divided platinum, palladium, nickel, or rhodium, two molar equivalents of hydrogen add to the triple bond of an alkyne to yield an alkane of same number of carbon atom.
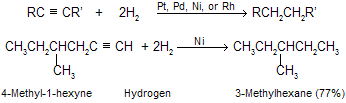
Substituents affect the heats of hydrogenation of alkynes in the same way that they affect in alkenes. Alkyl groups release electrons to sp hybridized carbon, stabilizing the alkyne and decreasing the heat of hydrogenation. For example, 1-butyne have higher heat of hydrogenation than 2-butyne.
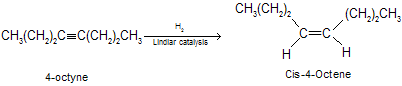
The heats of hydrogenation of alkynes are somewhat greater than twice the heats of hydrogenation of analogous alkenes.Alkenes are intermediates in the hydrogenation of alkynes lead us to consider the possibility of stopping hydrogenation at the alkene stage. It convert alkynes to alkenes by semihydrogenation in the presence of specially developed catalysts. The most frequently used is the Lindlar catalyst, a palladium on calcium carbonate combination with lead acetate and quinoline. Lead acetate and quinoline partially deactivate (“poison”) the catalyst, making it a poor catalyst for alkene hydrogenation while retaining its ability to catalyze the addition of hydrogen to alkynes.
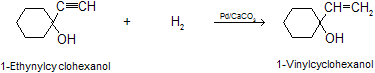
There are many other catalyst used for semihydrogenation of alkynes. These include palladium supported on barium sulphate, and a “nickel boride” Catalyst prepared by reaction of nickel salts with sodium borohydride (P–2, Ni2B). Hydrogenation of alkynes to alkenes by lindlar’s catalyst is highly stereoselective and yields the cis (for Z) alkene by syn addition to the triple bond.
Other alternative method of catalytic partial hydrogenation for converting alkynes to alkenes is reduction by a group first metal (lithium, sodium, or potassium) in liquid ammonia as the reaction medium. The metal- ammonia reduction is that it converts alkynes to trans (or E) alkenes. Thus, from the same alkyne one can prepare either a cis or a trans alkene by choosing the appropriate reagent.
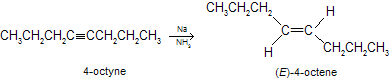
(iii) Hydration of alkynes (Kocharov’s reaction)
Addition of water in the presence of dil H2SO4 and HgSO4 is known as hydration of alkyne where a special kind of alcohol is formed in which the hydroxyl group is attached with doubly bonded carbon atom. This type of alcohol is called an enol (the double bond suffix -ene plus the alcohol suffix -ol). An important property of enols is their rapid tautomerization to aldehydes or ketones under the conditions of their formation due to stability.
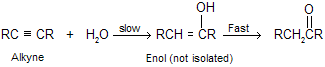
This reaction do not give good yield with ethyne because intermediate vinylic carbocation is unstable. The process by which enols are converted to aldehydes or ketones is called keto–enol isomerism or tautomerism which we already studied in isomerism, this is followed by the sequence of proton transfers shown in mechanism. Proton transfer to the double bond of an enol occurs readily because the carbocation that is produced is a very stable one. The positive charge on carbon is stabilized by electron release from oxygen back bonding and may be represented in terms of resonance resonating structure in following way.
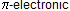 cloud from alkyne which increases reactivity for addition (same as cyclic mercurium species).
cloud from alkyne which increases reactivity for addition (same as cyclic mercurium species).Overall reaction mechanism:
Step A: Formation of enol takes place in aqueous acidic solution. The first phase of its transformation to a ketone is proton transfer to the carbon-carbon double bond.
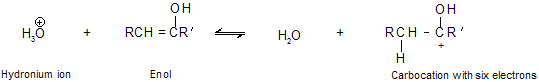
Step B: The carbocation stabilize by backbonding of electron to form ketones.