(viii) Reaction with HNO2. (Nitrosation of aliphatic amines)
(viii) Reaction with HNO2. (Nitrosation of aliphatic amines)When sodium nitrite reacts with H
2SO
4 or HCl it produces nitrous acid (HNO
2) which works as precursor of nitrosyl cation (N
+ O) this species is known as nitrosating agents.
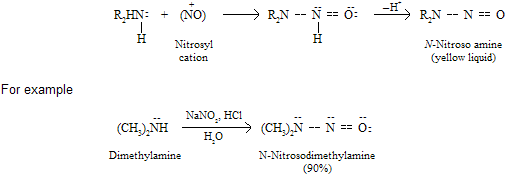
When any secondary amines reacts with nitrous acid, amine work as nucleophile which is attacking at nitrosyl cation to form yellow liquid nitroso solution.
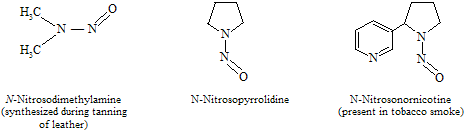
Some other examples of nitroso products
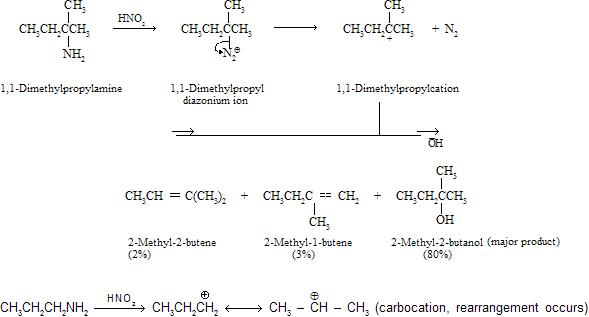
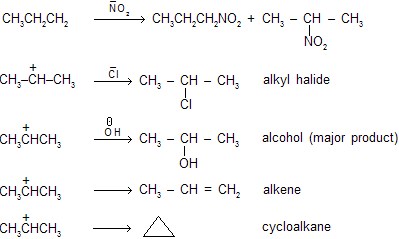
When primary amines undergo process of nitrosation, nitroso compounds formed first and can react further and finally produce various products where alcohols are formed as major product.
Alkyl diazonium ion is the final product of this sequence but not any other species can be isolated. When any primary amine is converted into diazonium species, it is known as diazotisation of amines. These are not very much stable due to positively charged nitrogen atom which is strained species. It’s decompose rapidly where molecular nitrogen is a leaving group and carbocations are formed.
As the nitrogen free products result from the formation and decomposition of diazonium salts so these reactions are known as deamination reactions.
The nitrosation of tertiary alkylamines is rather complicated, and these reactions are also not very useful in general chemistry primarilly they form addition product which breakdown on heating and form nitrasoamine.
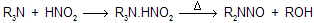

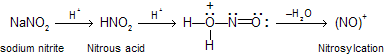
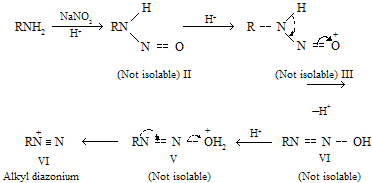
![]()