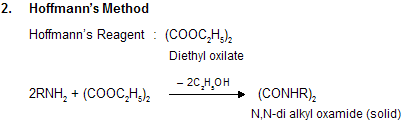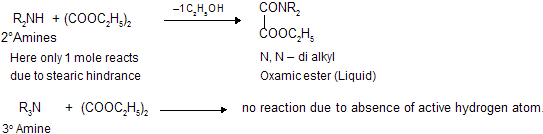Amines-Urea-4
(b) Nitrosation of aromatic amines
When aromatic amines undergo process of nitrosation by nitrosyl cation, electrophilic aromatic substitution occurs and then nitroso derivatives are formed. For example:
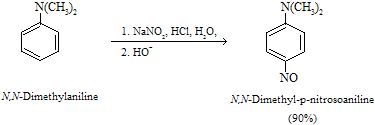
Only very strong activating group, for example aniline and its derivatives can react with very weak electrophile nitrosyl with nitrosyl cation it resemble with secondary amines where synthesis of N-alkyl nitrosoproduct takes place.
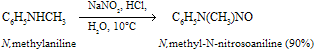
![]()
Aryl diazonium ions undergo a variety of reactions which makes them versatile intermediates for the preparation of many ring substituted aromatic derivatives for example
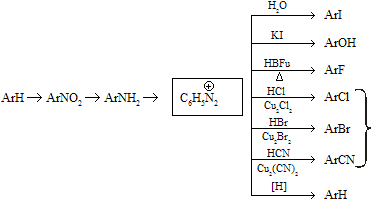
These reactions occur only when amino group attached directly with benzene ring but when benzyl derivatives type compounds undergo these reactions they form normal aliphatic amines type products.
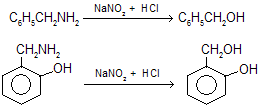
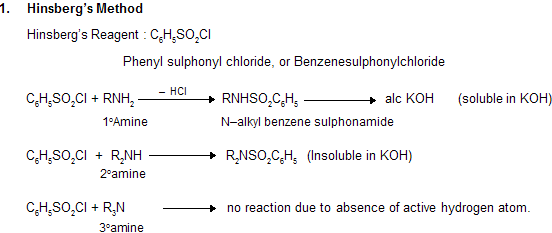
(x) Separation of 1o, 2o and 3o Amines