The following characteristics of the positive rays were recognised: - The rays travel in straight lines and cast a shadow of the object placed in their path.
- Like cathode rays, these rays also rotate the wheel placed in their path and also have heating effect. Thus, the rays possess kinetic energy, i.e., mass particles are present.
- The rays produce flashes of light on zinc sulphide screen.
- The rays are deflected by electric and magnetic fields in a direction opposite to that of cathode rays. These rays are attracted towards the negatively charged plate showing thereby that these rays carry positive charge.
- These rays can pass through thin metal foils.
- These rays can produce ionisation in gases.
- These rays are capable of producing physical and chemical changes.
- Positive particles in these rays have e/m values much smaller than that of electron. This means either m is high or the value of charge is small in comparison to electron. Since positive particle is formed by the loss of electron or electrons, the charge on the positive particle must be an integral multiple of the charge present on the electron. Hence, for a smaller value of e/m, it is definite that positive particles possess high mass.
- e/m value is dependent on the nature of the gas taken in the discharge tube, i.e., positive particles are different in different gases.
Accurate measurements of the charge and the mass of the particles obtained in the discharge tube containing hydrogen, the lightest of all gases, were made by J.J. Thomson in 1906. These particles were found to have the e/m value as + 9.579 × 104 coulomb/g. This was the maximum value of e/m observed for any positive particle. It was thus assumed that the positive particle given by hydrogen represents a fundamental particle of positive charge. This particle was named proton by Rutherford in 1911. Its charge was found to be equal in magnitude but opposite in sign to that of electron. Thus, proton carries a charge + 1.602 × 10--19 coulomb, i.e., one unit positive charge. The mass of the proton, thus, can be calculated. Mass of the proton = 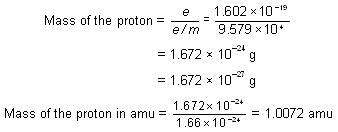
A proton is defined as a sub-atomic particle which has a mass nearly 1 amu and a charge of +1 unit (+1.602 × 10--19 coulomb). Protons are produced in a number of nuclear reactions. On the basis of such reactions, proton has been recognised as a fundamental building unit of the atom.
4.Rutherford Experiment-- Discovery of Nucleus After the discovery of electron and proton, the question arose how these charged particles are distributed in an atom. The answer was given by J.J. Thomson in the form of first model of the atom. He proposed that the positive charge is spread over a sphere in which the electrons are embedded to make the atom as a whole neutral. This model was much like resins in a pudding and is also known as Thomson's plum pudding model. This model was discarded as it was not consistent with the results of further investigations such as scattering of a-particles by thin metal foils. In 1911, Ernst Rutherford and his co-workers carried out a series of experiments using  -particles* (Fig. 3 and 4). A beam of -particles* (Fig. 3 and 4). A beam of  -particles was directed against a thin foil of about 0.0004 cm thickness of gold, platinum, silver or copper respectively. The foil was surrounded by a circular fluorescent zinc -particles was directed against a thin foil of about 0.0004 cm thickness of gold, platinum, silver or copper respectively. The foil was surrounded by a circular fluorescent zinc
*The radiations emitted by radioactive substances consist of  -particles. These particles are positively charged. These particles are actually helium atoms from which electrons have been removed. Each -particles. These particles are positively charged. These particles are actually helium atoms from which electrons have been removed. Each  -particle consists of a mass equal to about 4 times that of hydrogen atom and carries a positive charge of two units. It is represented by the symbol a or 42He. -particle consists of a mass equal to about 4 times that of hydrogen atom and carries a positive charge of two units. It is represented by the symbol a or 42He.
 -particles are usually obtained from a natural isotope of polonium-214. sulphide screen. Whenever an a-particle struck the screen, it produced a flash of light. The following observations were made: - Most of the
 -particles (nearly 99%) went straight without suffering any deflection. -particles (nearly 99%) went straight without suffering any deflection. - A few of them got deflected through small angles.
- A very few (about one in 20,000) did not pass through the foil at all but suffered large deflections (more thant 90°) or even came back in more or less the direction from which they have come, i.e., a deflection of 180°.
Consider an  -particle of mass `m' moving directly towards a nucleus with velocity -particle of mass `m' moving directly towards a nucleus with velocity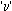 at any given time. As this at any given time. As this 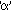 particle approaches the nucleus, its velocity and hence kinetic energy continues to decrease. At a certain distance r0 from the nucleus, the particle approaches the nucleus, its velocity and hence kinetic energy continues to decrease. At a certain distance r0 from the nucleus, the  -particle will stop and then start retracing its path depicted. This distance is called the distance of closest approach. At this distance, the kinetic energy of the -particle will stop and then start retracing its path depicted. This distance is called the distance of closest approach. At this distance, the kinetic energy of the  -particle is transformed into electrostatic potential energy. If Z be the atomic number of the nucleus then -particle is transformed into electrostatic potential energy. If Z be the atomic number of the nucleus then 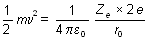
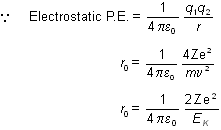
where, EK is the original kinetic energy of the a-particles Here, 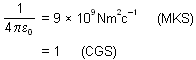
The distance of closest approach is of the order of 10-14 m. So, the radius of the nucleus should be less than 10-14 m. Following conclusions were drawn from the above observations: - Since most of the a-particles went straight through the metal foil undeflected, it means that there must be very large empty space within the atom or the atom is extra-ordinarily hollow.
- A few of the
 -particles were deflected from their original paths through moderate angles; it was concluded that whole of the positive charge is concentrated and the space occupied by this positive charge is very small in the atom. When -particles were deflected from their original paths through moderate angles; it was concluded that whole of the positive charge is concentrated and the space occupied by this positive charge is very small in the atom. When  -particles come closer to this point, they suffer a force of repulsion and deviate from their paths. -particles come closer to this point, they suffer a force of repulsion and deviate from their paths.
The positively charged heavy mass which occupies only a small volume in an atom is called nucleus. It is supposed to be present at the centre of the atom. 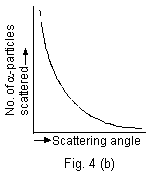 A very few of the A very few of the  -particles suffered strong deflections or even returned on their path indicating that the nucleus is rigid and -particles suffered strong deflections or even returned on their path indicating that the nucleus is rigid and  -particles recoil due to direct collision with the heavy positively charged mass. -particles recoil due to direct collision with the heavy positively charged mass.
The graph between angle of scattering and the number of a-particles scattering in the corresponding direction is as shown in Fig. 4(b).
Rutherford concluded that the number N of particles scattered at an angle  is such that is such that
N IMG
5. Moseley Experiment���Atomic Number Roentgen, in 1895, discovered that when high energy electrons in a discharge tube collide with the anode, penetrating radiations are produced which he named X-rays (Fig. 5).
X-rays are electromagnetic radiations of very small wave-lengths (0.1–20 Å). X-rays are diffracted by diffraction gratings like ordinary light rays and X-ray spectra are, thus, produced. 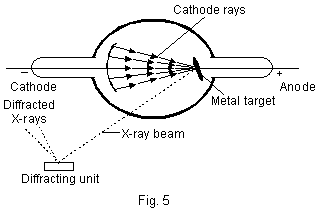 Each such spectrum is a characteristic property of the element used as anode.
Moseley (1912–13), investigated the X-ray spectra of 38 different elements, starting from aluminium and ending in gold. He measured the frequency of principal lines of a particular series (the a-lines in the K series) of the spectra. It was observed that the frequency of a particular spectral line gradually increased with the increase of atomic mass of the element. But, it was soon realised that the frequency of the particular spectral line was more precisely related with the serial number of the element in the periodic table which he termed as atomic number (Z). He presented the following relationship.
√v=a(Z-b)
where 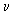 = frequency of X-rays, Z = atomic number, a and b are constants. When the values of square root of the frequency were plotted against atomic numbers of the elements producing X-rays, a straight line was obtained [Fig. 6]. = frequency of X-rays, Z = atomic number, a and b are constants. When the values of square root of the frequency were plotted against atomic numbers of the elements producing X-rays, a straight line was obtained [Fig. 6].
van den Broek (1913) pointed out that the atomic number of an element is equal to the total positive charge contained in the nucleus of its atom. Rutherford was also having the same opinion that the atomic number of an element represents the number of protons in the nucleus of its atom. Thus,
Atomic number of the element
= Serial number of the element in periodic table
= Charge on the nucleus of the atom of the element
= Number of protons present in the nucleus of the atom of the element
= Number of extranuclear electrons present in the atom of the element
6. Discovery of Neutron The discovery of neutron was actually made about 20 years after the structure of atom was elucidated by Rutherford. Atomic masses of different atoms could not be explained if it was accepted that atoms consisted only of protons and electrons. Thus, Rutherford (1920) suggested that in an atom, there must be present at least a third type of fundamental particles which should be electrically neutral and possess mass nearly equal to that of proton. He proposed the name for such fundamental particle as neutron. In 1932, Chadwick bombarded beryllium with a stream of  -particles. He observed that penetrating radiations were produced which were not affected by electric and magnetic fields. These radiations consisted of neutral particles, which were called neutrons. The nuclear reaction can be shown as: -particles. He observed that penetrating radiations were produced which were not affected by electric and magnetic fields. These radiations consisted of neutral particles, which were called neutrons. The nuclear reaction can be shown as: 49Be + 24He 612C + 01n 612C + 01n The mass of the neutron was determined. It was 1.675 × 10-24 g, i.e., nearly equal to the mass of proton. Thus, a neutron is a sub-atomic particle which has a mass 1.675 × 10-24 g, approximately 1 amu, or nearly equal to the mass of proton or hydrogen atom and carrying no electrical charge. The e/m value of a neutron is thus zero.
7. Rutherford Model On the basis of scattering experiments, Rutherford proposed a model of the atom which is known as nuclear atomic model. According to this model: - An atom consists of a heavy positively charged nucleus where all the protons and neutrons are present. Protons and neutrons are collectively referred to as nucleons. Almost whole of the mass of the atom is contributed by these nucleons. The magnitude of the positive charge on the nucleus is different for different atoms.
- The volume of the nucleus is very small and is only a minute fraction of the total volume of the atom. Nucleus has a diameter of the order of 10-12 to 10-13 cm and the atom has a diameter of the order of 10-8 cm.
Thus, diameter (size) of the atom is 100,000 times the diameter of the nucleus*.
The radius of a nucleus is proportional to the cube root of the number of nucleons within it.
R = R0A1/3cm
where R0 = 1.33 × 10-13; A = mass number; R = Radius of the nucleus
Rutherford and Marsden calculated the density of the hydrogen nucleus containing only one proton.
- There is an empty space around the nucleus called extranuclear part. In this part electrons are present. The number of electrons in an atom is always equal to number of protons present in the nucleus. As the nucleus part of the atom is responsible for the mass of the atom, the extranuclear part is responsible for its volume. The volume of the atom is about 1015 times the volume of the nucleus.
- Electrons revolve round the nucleus in closed orbits with high speeds. The centrifugal force acting on the revolving electrons is being counter balanced by the force of attraction between the electron and the nucleus.
This model was similar to the solar system, the nucleus representing the sun and revolving electrons as planets. The electrons are, therefore, generally referred to as planetary electrons.
|