Atomic - Structure-7: Ground state electron configuration for chromium
The above method of writing the electronic configurations is quite cumbersome. Hence, usually the electronic configuration of the atom of any element is simply represented by the notation.:
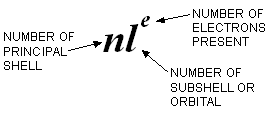
To get the complete configuration of an atom, a number of such notations are written one after the other in order of increasing energies of the orbitals, starting always with the orbital of lowest energy i.e. 1s.
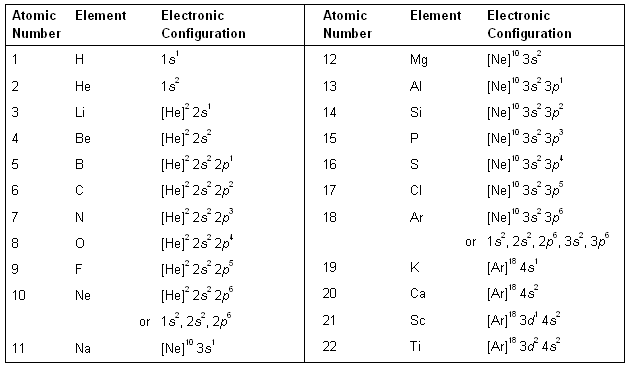
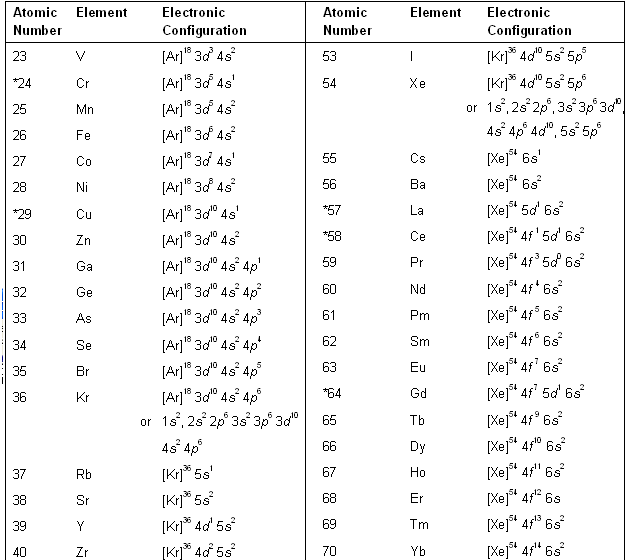
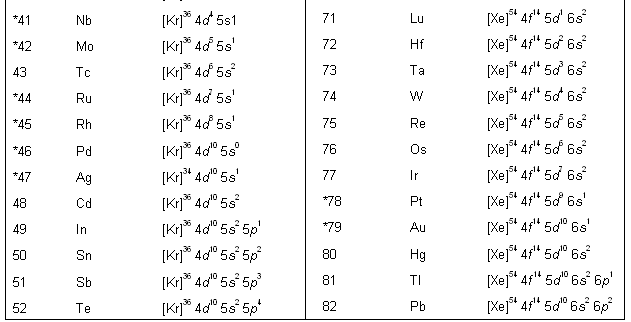
Using the above method of representation, the electronic configurations of the various elements are listed in table below.
Some exceptional electronic configurations. Some elements such as chromium (At. No. 24), copper (At. No. 29) etc. possess electronic configurations different from those expected from the aufbau order. This is because of the tendency of the sub-shells to be exactly half-filled or completely filled.
To illustrate this point, a few examples are given below :

Thus generally only one electron jumps from lower energy orbital to higher energy orbital e.g. from 4s to 3d. However in case of palladium, two electrons are involved (the only case with a difference.
The reason for the tendency of the subshells to be completely filled or exactly half-filled is that it leads to greater stability.
Cause of greater stability of exactly half-filled and completely filled configurations. The greater stability of these configurations is due to the following two reasons :
(i) Symmetry. The half-filled and completely filled configurations are more symmetrical and symmetry leads to greater stability.
(ii) Exchange energy. The electrons present in the different orbitals of the same subshell can exchange their positions. Each such exchange leads to a greater stability which can be explained in terms of exchange energy. As the number of exchanges that can take place is maximum in the exactly half-filled and completely filled arrangements (i.e. more in d5 than in d4 and more in d10 than in d9), therefore exchange energy is maximum and hence the stability is maximum.
Some important points in writing electronic configurations. While writing the electronic configurations, the following points may also be noted:
(i) To avoid the writing of electronic configurations in a lengthy way, usually the symbols [He]2, [Ne]10, [Ar]18 etc. are used as the first part of the configuration. Such a symbol stands for the electronic configuration of that inert gas and is usually called the core of the inert gas.
(ii) Although the orbitals of lower energy are filled first but the electronic configuration are written not in the order in which the orbitals were filled but in the order of principal quantum numbers.
(iii) Unless otherwise mentioned, electronic configuration always means the electronic configuration in the ground state.
(iv) Always remember that if you write down electronic configuration of ion (cation or anion), than first you write configuration of basic atom than add or remove the electron from the system otherwise always there is a chance of error. For example
| (1) Cr+3, First Cr 3d5 4s1 than Cr+3 |
For elements with very high atomic numbers, some deviations are observed other than on account of half-filled and fully filled subshells. However, for our purposes, such exceptions are not important. Table. Electronic configuration of elements in the ground state