Atomic Structure - 1
The most special thing about this chapter is that the no. of scientists involved in developed of this concept is remarkably large. Starting with heucippus of Miletus in 440 BC it extends to 2000's. And the research is still going on. Even the great scientist EINSTEIN had his hands in developing this extraordinary concept.
Atomic No.: Total no. of protons present in nucleus.
Mass No.: Total no. of protons & Neutrons in neucleus.
Atomic No.(Z): no. of electrons (when atom is neutral).
Mass No.(A): no. of neutrons + no. of protons.
Representation 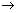
Ilustration: Calculate no. of protons, neutrons & electrons in 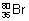 .
.
Ans: Z = 35, A = 80 No. of protons = Z = 35
No. of protons = Z = 35
No. of neutrons = A - Z = 80 - 35 = 45
As atom is neutral. No. of electrons = No. of protons = 35.
Isotopes: Such atoms of same element having same atomic no. but different mass no.s are c/d isotopes.
Protiun (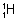 ). Denterium (
). Denterium (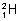 ) & Tritium (
) & Tritium (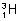 ) are isotopes.
) are isotopes.
Isobars: Atoms of different elementswhich have same mass no.s but different atomic No.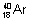 ,
, 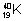 ,
, 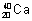 are isobars.
are isobars.
Isotones: Atomes of different elements which contain same no. of neutrons c/d isotones.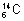 ,
, 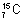 are isotones because they have same no. of neutrons.
are isotones because they have same no. of neutrons.
subatomic particle like electron at high speed varies.
m = 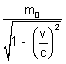
m0 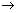 restmass
restmass
c 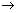 velocity of light
velocity of light
Photoelectric effect: When radiations with certain minimum frequency (v0) strike surface of metal electrons are ejected from surface of metal. This is c/d photoelectric effect & electrons are called photoelectrons.
Threshold frequency: It is min. frequency (v0) below which no electrons are ejected.
Work function: Min. energy required to eject electron (h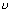 0).
0).
h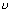 = h
= h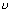 0 +
0 + 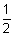 mv2
mv2
Total energy = Workfunction + Kinetic energy.
Planck's Quantam Theory:
(i) Radiant energy is emitted or absorbed not continuously but discontinuously in form of small discrete packets of energy. Each packet c/d 'quanta'. In case of hight energy 'quanta' is 'photon'.
(ii) Energy of each quantum is directly proportional to frequency of radiation.
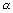
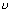
E = h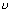
h  Planck's Constant.
Planck's Constant.
h = 6.626 x 10-34 J/sec.
Body will be some whole no. quanta.
E = nh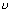
n 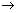 integer.
integer.
Illustration: Calculate KE of electrons ejected when yellow light of frequency 5.2 x 1014 s-1 on a metal whose threshold frequency is 4 x 1014 s-1.
Ans: h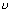 = h
= h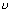 0 +
0 + 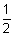 mv2
mv2
h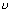 - h
- h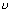 0 =
0 = 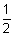 mv2
mv2 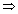
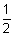 mv2 = h(1.2 x 1014)
mv2 = h(1.2 x 1014)
= 6.625 x 10-34 x 1.2 x 1014
= 7.95 x 10-20 J
Bohr's Model:
Postulate:
(1) As long as electrons occupy definite energy level, it does not radiate out energy. Emission or absorption of energy occures only when electrons jumps from one level to other.
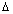 E = En2 - En1 = h
E = En2 - En1 = h
If n2 > n1 emission epectra.
If n2 < n1 absorption spectra.
n1 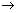 Initial state.
Initial state.
n2  Final state.
Final state.
(2) Angular momentum of electron in closed is always quantized i.e. integer multiple of 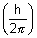 .
.
Angular momentum = n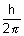
mvr = n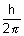 .
.
Dumb Question: Why angular momentum is mvr ?
Ans: Angular momentum = Moment of Inertia x angular velocity.
= 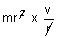 = mvr
= mvr
Note: In CGS unit, 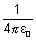 = 1
= 1
MKS unit, 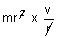 = 9 x 109 Nm2c-2.
= 9 x 109 Nm2c-2.