Atomic Structure - 2
Calculation of radius of orbit:
Derivation:
Electrons revolves in orbit.
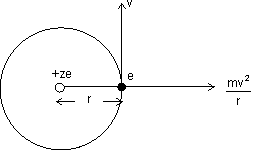
Centripetal force acting on electron away from centre & force of attraction towards centre.
For electron to revolve in same orbit.
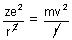 [
[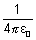 = 1 in CGS unit] ....................... (i)
= 1 in CGS unit] ....................... (i)mvr = n
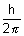 ....................................................... (ii)
....................................................... (ii)v =
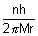
Putting value of v from eq. (ii) in eq. (i)
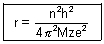 .................................................... (iii)
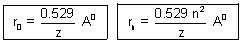
.................................................... (iii)r0 =
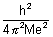 = 0.529 A0
= 0.529 A0 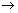 Bohr radius
Bohr radius| For H-atom, rn = n2 x r0 = n2 x 0.529 A0 |
For H-like atom, like He+,
Calculation energy of electron:
Total energy of electron (E) = K.E. + P.E.
=
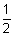 mv2 -
mv2 - 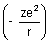
Dumb Question: Why P.E. is -
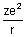 ?
?Ans: P.E. is work done when electron moves from
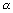 to r.
to r.P.E. =
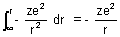
Dumb Question: Why Force is -ve ?
Ans: Force is work attractive. So, it is taken as -ve.
From eq. (i) mv2 =
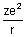
E =
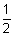
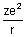 -
- 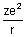 = -
= - 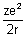
Dumb Question: What does -ve sign signify ?
Ans: -ve sign show's that electron is bound to that orbit & atom.
E = -
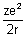
Substituting value of r from eq. (iii)
..................
Calculation of velocity of electron in any orbit:
Substituting value of r from (ii) in (i)
v =
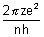
For H-like atom,
vn =
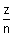 x 2.188 x 108 cms-1
x 2.188 x 108 cms-1For H-atom, putting z = 1
vn =
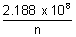 cms-1
cms-1From eq. (i)
v2 =
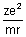
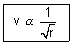
Calculation of no. of revolutions of electron in an orbit per sec:
mvr = n
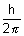
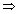 v =
v = 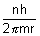
No. of rev./sec =

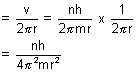
No. of revolutions per sec =
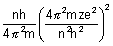
=
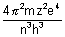
Calculation of no. of waves in any orbit:
No. of waves in any orbit =
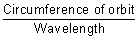
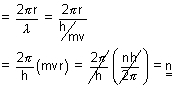
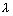 =
= 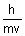
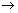 De Broglie relation.
De Broglie relation.Waver no.: It is reciprocal of wavelength.
For H-atom (wave no.)
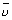 = R
= R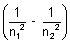 R
R 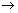 Rydberg constant
Rydberg constantR = 1.097 x 107 m-1
For Lyman Series n1 = 1, n2 = 2, 3, 4, .....................................
For Balmer Series n1 = 2, n2 = 3, 4, 5, .....................................
For Paschen Series n1 = 3, n2 = 4, 5, 6, .....................................
For Brackett Series n1 = 4, n2 = 5, 6, 7, .....................................
For Pfund Series n1 = 5, n2 = 6, 7, 8, .....................................
For H-like atom,
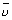 = R
= R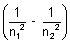 z2
z2z - atomic No.
when n2 in Redbergis formula is
 i.e. n2 =
i.e. n2 = 
De Broglie Relation:
Matters have dual nature of particle & wave If assumed as wave, its energy.
E = h
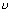
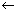 Plank's quantum theory .................................... (i)
Plank's quantum theory .................................... (i)If assumed as particle, its energy.
E = mc2
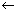 Einstein Eq. .................................................. (ii)
Einstein Eq. .................................................. (ii)Equating (1) & (2)
h
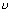 = mc2
= mc2sinc
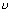 =
= 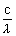
h
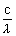 = mc2
= mc2 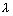 = = 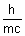 |
Deg broglie pointed that this eq. can be applicable to any particle.
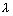 =
= 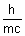 =
= 
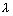 =
= 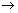 de broglie wavelength.
de broglie wavelength.