Atomic Structure - 3
Calculation of de broglie wavelength of electron from potential applied to accelerate it:
If accelerating potential v is applied,
Energy by electron = ev (charge x potential = ….)
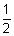 mv2 = ev
mv2 = ev 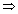
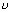 =
= 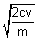
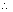
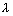 =
= 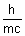 =
= 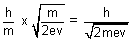
Illustration: Calculate wavelength of spectral line in spwectra of Li2+ ion when transition takes place b/w two levels whose sum is 6 & difference is 2.
Ans: Let transition takes place b/w levels n1 & n2
n1 + n2 = 6 & n2 - n1 = 2
On solving n2 = 4 n2 = 2
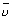 =
= 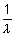 = R
= R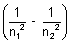 z2
z2
Here z = 3 (Li2+ ion)
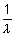 = 109,677 cm-1
= 109,677 cm-1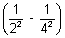 x 32
x 32
= 109,677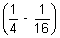 x 9
x 9
= 109,677 x 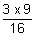
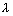 =
= 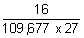
= 5.4 x 10-6
Illustration: Calculate (i) Ist excitation energy yo electron of He+ atm.
(ii) Ionization energy of He+ atom.
atom,
En = - 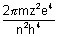
Dumb Question: What is Ist excitation energy ?
Ans: It is amount of energy required to excite electron from h = 1 (ground state) to n = 2 (Ist excite state)
 E = E2 - E1
E = E2 - E1
z = 2
E1 = 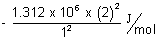 & E2 =
& E2 = 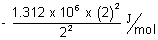
= - 1.312 x 106 + 4 x (1.312 x 6) = 3 x 1.312 x 102 J/mol
= 3.936 x 106 J/mol
(ii) Dumb Question: What is Ionization energy ?
Ans: Energy required to remove electron from n = 1 to n = 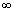 i.e.
i.e.
I.E. = 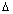 E =
E = 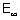 - E1 = 0 - (- 1.312 x 106 x 4)
- E1 = 0 - (- 1.312 x 106 x 4)
= 5.248 x 106 J/mol
Dumb Question: Why 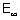 = 0 ?
= 0 ?
Ans: En 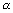
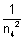 & when n
& when n 
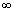
then 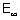
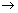 0
0
So, 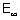 = 0
= 0
Question ….. with electron (m = 9.1 x 10-31 kg) moving with velocity 103 m/s & find refarding potential required to stop electron ?
Ans: 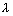 =
= 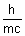 =
= 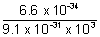 = 7.25 x 10-7 m
= 7.25 x 10-7 m
Let v be retarding potential in volt.
To stop electron, K.E> of electron = opposing energy of potential.
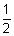 mv2 = ev
mv2 = ev
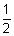 x 9.1 x 10-31 x 106 = 1.6 x 10-19 x v
x 9.1 x 10-31 x 106 = 1.6 x 10-19 x v
v = 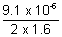 volt v = 2.844 x 10-6 volt
volt v = 2.844 x 10-6 volt
Derivation of Bohr’s Postulate of angular momentum from de boglie wavelength:
According to De Broglie electron is not only particle but has wave character.
So, circumference of orbit must be equal to integral nultiple of wavelength (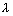 ) for com[letely in phase.
) for com[letely in phase.
i.e. 2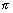 r = n
r = n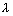 ……………………………… (i)
……………………………… (i)
r 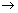 radius of orbit.
radius of orbit.
But 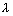 =
= 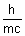
2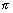 r =
r = 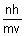
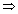 mvr =
mvr = 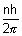
It is impossible to measure simultaneously position and momentum of a small particle with absolute accuracy.
Product of uncertainity in momentum (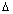 P = m
P = m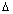 v) & position is constant & is equal to or greater than
v) & position is constant & is equal to or greater than 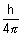 , where h
, where h 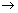 Planck’s constant.
Planck’s constant.
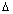 x.
x.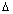 P
P 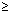
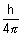
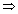
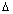 x.(m
x.(m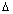 v)
v) 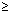
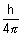
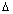 x.
x.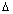 v
v 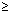
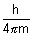
It has significance only for microscopic particles.
Dumb Question: Why electron can not exist in nucleus ?
Ans: Diameter of atomic nucleus is order of 10-15m.
So, max. uncertainity in its position would be 10-15m (i.e. 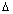 x = 10-15m)
x = 10-15m)
Mass of electron = 9.1 x 10-31
By uncertainity Principle
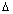 x.(m
x.(m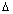 v) =
v) = 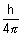
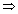
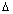 v =
v = 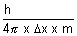
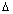 v =
v = 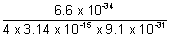
= 5.77 x 1010 m/s.
This value is much higher than velocity of light (3 x 108 m/s). So, it is not possible.
Question: ……… with uncertainity of 0.02%. What is uncertainity in position.
Ans: 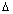 v =
v = 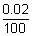 x 500 = 0.1 m/s
x 500 = 0.1 m/s
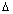 x.
x.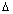 v =
v = 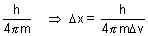
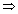
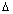 x =
x = 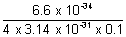 = 5.77 x 10-4 m
= 5.77 x 10-4 m