Atomic Structure - 4
Size of nucleus:
Size of nucleus (r) = (1.3 x 10-13) m1/3
r 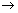 rad. of nucleus m
rad. of nucleus m 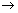 Atomic no.
Atomic no.
Dumb Question: Why radius of nucleus varies m1/3 i.e. r 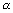 m1/3 ?
m1/3 ?
Ans: If nucleus isspherical,
density d = 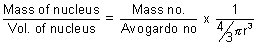
d = 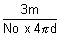
r3 = 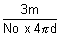
r = 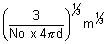
r = 1.3 x 10-13 x m1/3
r 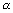 m1/3
m1/3
……………………..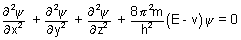
amplitude of wave 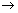
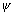
Coordinates of electron are (x, y, z), E is total energy of electron, v is its potential energy, m is mass of electron & h is planck’s constant.
Significance of wave function: Square of wave function (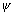 ) gives probability of finding electron at that point.
) gives probability of finding electron at that point.
Probability of finding electron = 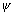 2
2
Quantum No.: Set of foor no.s with help of which we can get complete information about all electrons in an atom i.e., location, energy, shap & orientation of orbital.
Principal quantam No. (n):
(i) It gives average distance of electron from nucleus.
(ii) It completely determines energy of electron in H-atom & H-like atoms.
En = - 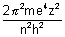
For Ist principal shell (k), n = 1 which means it has lowest energy & closest to nucleus.
(iii) Max. no. of elctrons present in any principal shell is given by 2n2 n  no. of principal shell.
no. of principal shell.
Question: ……………………
Ans:  is wave ………. schrodinger eq. It can be -ve or +ve but square of it always +ve & probability of finding electron at any place can not be -ve. So, it represents probability.
is wave ………. schrodinger eq. It can be -ve or +ve but square of it always +ve & probability of finding electron at any place can not be -ve. So, it represents probability.
(i) It gives no. of subshells present in main shell.
(ii) Angular momentum of electron present in any sub shell.
(iii) Shapee of various subshells present within some principal shell.
Angular momentum of electron =
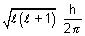

 Azimuthal quantum no.
Azimuthal quantum no.For given value of n, it have integral value ranging from 0 to n - 1.
For 1st shell, n = 1,
 = 0 & have only one subshell c/d s-subshell.
= 0 & have only one subshell c/d s-subshell.For IInd shell, n = 2,
 = 0 & 1 & have two subshell s-subshell (
= 0 & 1 & have two subshell s-subshell ( = 0)
= 0)& P-subshell (
 = 1)
= 1)For IIIrd shell, n = 3,
 = 0, 1, 2 & havethree subshell s, p & d - subshells. For d-subshell (
= 0, 1, 2 & havethree subshell s, p & d - subshells. For d-subshell ( = 1).
= 1).For IVth shell, n = 4,
 = 0, 1, 2, 3 & have 4 subshells s, p, d & f & for f subshell
= 0, 1, 2, 3 & have 4 subshells s, p, d & f & for f subshell(
 = 0)
= 0)Note: (1) No. of subshells present in any principal shell is equal to no. of principal quantum no.
(2) Energies of different subshells are found to be in order
s < p < d < f
(1) It gives no. of orbitals present in any subshell.
For given
 , m has in5tegral value -
, m has in5tegral value -  to +
to +  .
.For
 = 0, m has only one value i.e. m = 0
= 0, m has only one value i.e. m = 0This means s-subshell has only one orientation in space.
For
 = 1, m has 3 value i.e. m = - 1, 0 & + 1
= 1, m has 3 value i.e. m = - 1, 0 & + 1This means p-subshell has 3 orbitals. These 3 orbitals are oriented along x-axis, y & z-axis. i.e. Px, Py & Pz.
For
 = 2, m have 5 values i.e. m = - 2, -1, 0, + 1 & + 2 d-subshell has 5 orbitals.
= 2, m have 5 values i.e. m = - 2, -1, 0, + 1 & + 2 d-subshell has 5 orbitals.For
 = 3, m has 7 value, So, has 7 orbitals.
= 3, m has 7 value, So, has 7 orbitals.Note: By convention, for Pz, m = 0, & for Px & Py m ± 1 but not fixed.
Five d-orbitals. For dz2, m = 0, for dxy & dx2 - y2, m = ± 1.
Degenerate orbitals: Those orbitals which have same subshell having equal energy.
(4) Spin Quantum No.:
(1) To explain magnetic properties of substance.
(2) Direction of electron spin i.e. clockwise or anticlockwise.
For any valve of m, s = + 1/2 or - 1/2
Illustration: An electron is in 4f orbital. What possible values for quantum numbers n,
 , m & s can it have ?
, m & s can it have ?Ans: Since electron is in 4f orbital, value of principal quantum no., n = 4
For f-orbital, Azimuthal quantum no.
 = 3. Values of magnetic quantum (m) are -
= 3. Values of magnetic quantum (m) are -  to +
to +  in which zero.
in which zero. = 3, m = - 3, -2, -1, 0, +1, +2, +3
= 3, m = - 3, -2, -1, 0, +1, +2, +3For each value of m, spin quantum no., s has two values, i.e., s = + 1/2 and s = - 1/2.
Pauli Exclusion Principle:
 (1) No two electrons in an atom can have same set of four quantum numbers.
(1) No two electrons in an atom can have same set of four quantum numbers.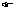 (2) An orbital can have maximum two electrons & these must have opposite spins.
(2) An orbital can have maximum two electrons & these must have opposite spins.Illustration: Let for 3s-orbital, It has n = 3,
 = 0 & m = 0. Since for each value of m, there are two values, spin quantum no. i.e. +
= 0 & m = 0. Since for each value of m, there are two values, spin quantum no. i.e. + 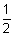 and -
and - 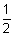 . 3-s orbital can have two electrons; one with quantum no. n = 3,
. 3-s orbital can have two electrons; one with quantum no. n = 3, = 0, m = 0 & s = +
= 0, m = 0 & s = + 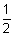
& other n = 3,
 = 0, m = 0 & s = -
= 0, m = 0 & s = - 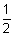
No. of orbitals in subshell = 2
 + 1
+ 1Max. no. of electrons in subshell = 2(2
 + 1)
+ 1)No. of orbitals in nth shell = n2
Max. no. of electrons in nth shell = 2n2
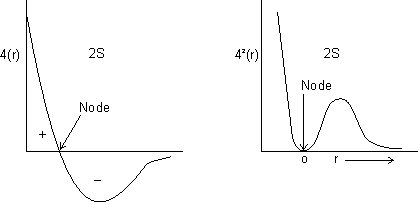
Shape of s-orbitals
Shape of s-orbital is spherical.
For 1s, PRobability of electron is max. near nucleus &
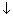 as distance
as distance 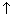 .
.For 2s, Prob. of electron is max. neaqr nucleus & then
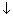 to zero &
to zero &  again & then
again & then  as distance from nucleus
as distance from nucleus  .
.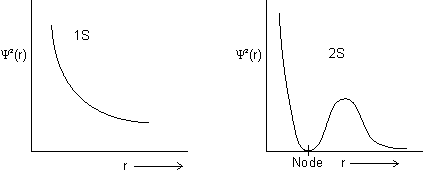
Dumb Question: What is node ?
Ans: It is region where probability of finding electron is zero c/d node.