Atomic Structure - 7
Q.3. Wave function of 2s electron is given by 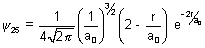 . It has node at r = r0. Find relation b/w r0 & a0.
. It has node at r = r0. Find relation b/w r0 & a0.
Ans: 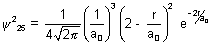
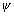 225 = 0 at r = r0
225 = 0 at r = r0
[Dumb Question: Why 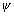 225 or prob. of finding electron is zero ?
225 or prob. of finding electron is zero ?
Ans: At r = r0, it has node where probability of finding electron is zero.]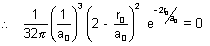
In this expression, only factor can be zero is 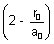
2 - 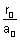 = 0
= 0  r0 = 2a0
r0 = 2a0
Q.4. A dye absorbs light of 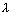 = 4530 A0 & then fluorescence light of 50870 A0. 47% of absorbed energy is reemitted out as flurescence. Calculate ratio of quanta emitted out to no. of quanta absorbed.
= 4530 A0 & then fluorescence light of 50870 A0. 47% of absorbed energy is reemitted out as flurescence. Calculate ratio of quanta emitted out to no. of quanta absorbed.
Let n1 photons areabsorbed. 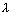 absorbed
absorbed
Total energy absorbed = 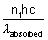
E of light re-emitted out in one photon = 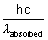
Let n2 photons are reemitted then,
Total energy reemitted out = 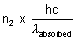
As given
Eabsorbed x 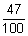 = Ereemitted out
= Ereemitted out
 x
x 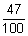 = n2 x
= n2 x 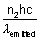
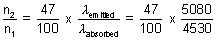
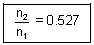
Dumb Question: What is fluorescence ?
Ans: Emission of radiation from particle ?
Q.5. Waht H-like ion has wavelength difference b/w Ist lines of Balmer & Lyman series equal to 59.3 nm RH = 109678 cm-1
Ans: 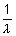 = RHz2
= RHz2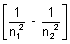
For I line of Balmer series
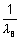 = RHz2
= RHz2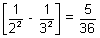 x RH x z2
x RH x z2
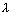 B =
B = 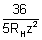
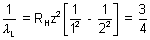 RHz
RHz
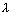 L =
L = 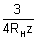
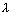 B -
B - 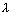 L = 59.3 x 10-7 cm.
L = 59.3 x 10-7 cm.
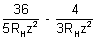 = 59.3 x 10-7
= 59.3 x 10-7
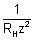 [7.2 - 1.333] = 59.3 x 10-7
[7.2 - 1.333] = 59.3 x 10-7
z2 = 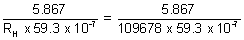
z = 3
H-like atom is Li2+.