Atomic Structure - 8
HARD TYPE
Q.1. 1.8g H - atoms are excited to radiation. Spectra shows that 27% of atoms are in IIIrd energy level & 1.5% of atoms in IInd energy level & rest in ground state. Ionization potential of H is 13.6 ev. Calculate
(i) No. of atoms present in III, II energy level.
(ii) Total energy envolved when all atoms return to ground state.
Ans: 1g H contains = No atoms
1.8g H contains = 6.023 x 1023 x 1.8
= 10.84 x 1023 atoms
(a)
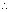 No. of atoms in III shell =
No. of atoms in III shell = 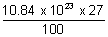
= 292.68 x 1021 atoms.
=
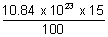 = 162.6 x 1021 atoms.
= 162.6 x 1021 atoms.(b) When all atoms return ti I shell, then
E' = (E3 - E1) x 292.68 x 1021
En = -
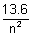 ev
evE' = (-
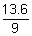 + 13.6) x 292.68 x 1021 x 1.6 x 10-19 [
+ 13.6) x 292.68 x 1021 x 1.6 x 10-19 [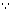 1 ev = 1.6 x 10-19 J]
1 ev = 1.6 x 10-19 J]= 5.668 x 105 J
E'' = (E2 - E1) x 162.6 x 1021
= (-
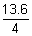 + 13.6) x 1.6 x 10-19 x 162.6 x 1021
+ 13.6) x 1.6 x 10-19 x 162.6 x 1021= 2.657 x 105 J
E = E' + E'' = (5.668 + 2.657) x 105 J
Total energy envolved = 832.5 KJ
Q.2. Find quantum no. 'n' corresponding to excited state of He+ ion if on transitiontoground state that ion emits two photons in succession with wavelengths 18.5 & 30.4 nm respectively.
Ans: Suppose electron in excited state is present in n2 shell. First it falls from n2 to n1 & then from n1 to ground state (n = 1).
(i) n2
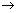 n1
n1 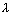 1 = 108.5 nm
1 = 108.5 nm(ii) n1
 G.State,
G.State, 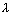 2 = 30.4 nm
2 = 30.4 nm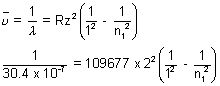
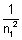 = 1 - 0.75
= 1 - 0.75 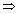 n12 =
n12 = 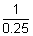
n1 = 2
Again,
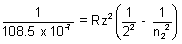
= 109677 x 22
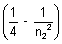
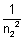 = 0.25 - 0.21 = 0.04
= 0.25 - 0.21 = 0.04n22 =
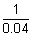 = 25
= 25  n2 = 5
n2 = 5Q.3. The wave function of orbital is given by
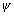 (r) =
(r) = 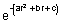 . Find thevalue of r for which probability of finding electron is max.
. Find thevalue of r for which probability of finding electron is max.Ans: Probability of finding electron
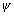 2(r) =
2(r) = 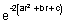
For max. probability finding electron
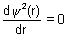 &
& 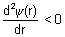
..................
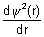 = e-c[
= e-c[ (-4ar)e-2br +
(-4ar)e-2br +  e-2br(-2b)]
e-2br(-2b)]= e-c
 e-2br[- 4ar - 2b]
e-2br[- 4ar - 2b]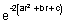 [- 4ar - 2b] = 0
[- 4ar - 2b] = 04ar = - 2b
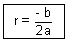
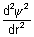 = (- a)
= (- a)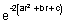 + (- 4ar - 2b)2
+ (- 4ar - 2b)2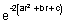
=
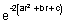 [- 4a + (4ar + 2b)2]
[- 4a + (4ar + 2b)2]=
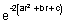 [- 4a + 16a2r2 + 4b2 + 16abr]
[- 4a + 16a2r2 + 4b2 + 16abr]Putting r = -
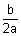
=
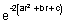 [- 4a + 16a2 x
[- 4a + 16a2 x 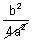 + 4b2 + 16ab x -
+ 4b2 + 16ab x -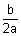 ]
]=
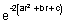 [- 4a +
[- 4a + 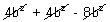 ]
]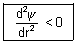
Max. prob. of finding electron
when r = -
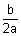
Key Words
 Bohr's Model of atom.
Bohr's Model of atom. Photoelectric effect.
Photoelectric effect. Emission Spectra.
Emission Spectra. Absorption Spectra.
Absorption Spectra. Rydberg's Formula.
Rydberg's Formula. De broglie Wavelength.
De broglie Wavelength. Heisen berg's Uncertainity Principle.
Heisen berg's Uncertainity Principle. Wave Function
Wave Function Quantum No. &
Quantum No. & Principal Quantum No.
Principal Quantum No. Azimuthal Quantum No.
Azimuthal Quantum No. Amgnetic Quantum No.
Amgnetic Quantum No. Spin Quantum No.
Spin Quantum No. Paulieexclusion Principle.
Paulieexclusion Principle. Nodal Plane.
Nodal Plane. Nund's Law.
Nund's Law. Aufbaw's Principle
Aufbaw's Principle