BOHR MODEL & X-RAYS - 1
Physics: Modern Physics
Neils Bohr gave forward his model of atom with answeres most of the flaws that Rutherford model had. This model introduced quantization principal and this model is regarded as first quantum mechanical model. We will explore in detail, this structure of atom.We will also look into X - ray formation which is a converse process of photoclectric effect and will examine Moreley's law in pantium.
THE BOHR THEORY OF HYDROGE ATOM (AND HYDROGEN LIKE ATOM)
Bohr gave following postulates for electron in hydrogen atom :-
• An electron in an atom could resolve in certain stable orbits without the emission of radiant energy.
• An electron resolves around the nucleus only in those orbits for which the angular momentum is some integral multiple of
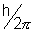 . Thus angular of electron is QUANTAIZED.
. Thus angular of electron is QUANTAIZED.L =
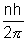 , where n
, where n • An electron might a transition from one of its specified non-radiating orbits to another of lower energy. When it does so, a single photon is amitted having energy equal to the energy difference between the initial and final slater.
The frequency of emitted photon is given by
h
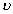 = Ei - Ef
= Ei - EfDumb Question:- Are these equations valid for all atoms ?
Ans:- No, equations are valid only for single electron system, i.e. systems where only one electron is present. Ideally this equation applies only to Hydrogen atom, Singly ionized helium He+, and doubly ionized Lithium Li+ +.
In Bohr's model, radius of nth orbit is given by rn =
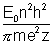 and speed of electron is given by Vn =
and speed of electron is given by Vn = if values of constant are evaluate then,
rn = 0.529 n2/z
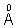 and Vn = 2.19 X 106
and Vn = 2.19 X 106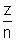 m/sec.
m/sec.where n
 Principal quantum number/shell number.
Principal quantum number/shell number.z
 atomic number of nucleus involved.
atomic number of nucleus involved.Why ??
from Bohr's postulate, angular momentum of electron in nth orbit is L =
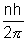 also from mechanics L = mvnrn
also from mechanics L = mvnrn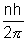 ............................................... (1)
............................................... (1)for electron to resolve in its orbit, Electrostatic force is providing the required centripetal force.
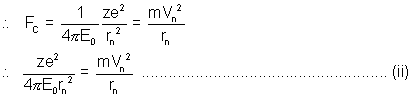
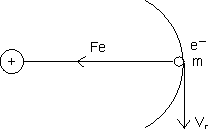
Solving (1) & (2) simultaneously yields the required result.