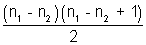BOHR MODEL & X-RAYS - 2
Bohr Energy levels:- The energy of a revolving electron is :-
(i) Kinetic Energy, due to its revolution in the orbit.
(ii) Electrostatic Potential Energy.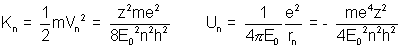
The total energy Eu is the sum of kinetic and potential energies.
Eu = Ku + Vu = - 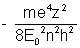
if the value of constants are placed then
Eu = 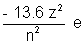
Dumb Question:- What is the significance of total energy of e- as a negative quantity?
Ans:- Total energy as negative quantity sindicates that it is held in a stable state and energy needs to be supplied to it if electron has to be pulled out.
Dumb Question:- Where has the zero of potential energy been let ?
Ans:- The zero of electrostatic potential energy has been setup at infinity i.e. outside the bounds of atom.
If an electron moves from ni orbit to lower nf orbit then it lose, energy which is emitted in the form of radiations and the wavelength of such radiation is given by formula.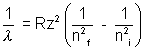 , Where R = Rydberg’s constant = 1.097 x 107 m- 1
, Where R = Rydberg’s constant = 1.097 x 107 m- 1
Why ??
energy of an orbit is given by En = - 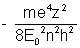
from Bohr’s postulate when an electron falls from higher orbit to lower orbit then energy of emitted radiation is given by E = Ei - Ef =
E = Ei - Ef = 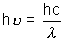
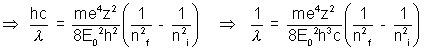
if R = 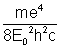 (constant)
(constant)
then 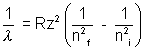
Few named transitions :-
Lymen Series 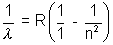 n = 2, 3, 4,……………
n = 2, 3, 4,……………
Balmen Series 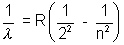 n = 3, 4, 5,……………
n = 3, 4, 5,……………
Paschen Series 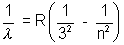 n = 4, 5, 6,……………
n = 4, 5, 6,……………
Brackett Series 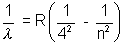 n = 5, 6, 7,……………
n = 5, 6, 7,……………
Pfund Series 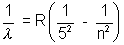 n = 6, 7, 8,……………
n = 6, 7, 8,……………
(In all these cases electron is moving down from nth orbit to concerned orbit)
• Total number of emission lines from same excited state n1 to another state n2 (< n1) is given by