BOHR MODEL & X-RAYS - 5
- Calculate the second excitation potential of helium. Given e = 1.6 X 10-18C,
m = 9.1 X 10-31Kg.
Solution:- Energy of first orbit, E1 = 36.6 z2
Energy of third orbit, E3 =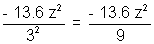
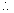 Third excilation potential = E3 - E1 = 13.6 z2(1 - 1/9)eV = 48.4 eV [for helium Z=2]
Third excilation potential = E3 - E1 = 13.6 z2(1 - 1/9)eV = 48.4 eV [for helium Z=2] - In Moseley’s equation
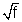 = a(z - b), a and b are constant. Find their values with the help of following data
= a(z - b), a and b are constant. Find their values with the help of following data
| Element | z | Wavelength of Kd X-ray. |
| M0 C0 | 42 27 | 0.71  1.785 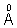 |
Solution:-
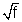 = a(Z - b) or
= a(Z - b) or 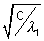 = a(Z1 - b) .................................... (i)
= a(Z1 - b) .................................... (i)and
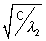 = a(Z2 - b) ........................................... (ii)
= a(Z2 - b) ........................................... (ii)from (i) & (ii) we have
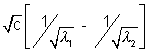 = a(z1 - z2) .......................................... (iii)
= a(z1 - z2) .......................................... (iii)Solving the 3 equations with C = 3 X 108m/s,
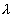 = 0.71 X 1070 and
= 0.71 X 1070 and 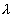 2 = 1.785 X 10-10m and Z1 = 42 and Z2 = 27, we get
2 = 1.785 X 10-10m and Z1 = 42 and Z2 = 27, we geta = 5 X 107(Hz)1/2 and b = 1.37
7. The wavelength of the first line of Lymen series for hydrogen is identical to that of the second line of Balmen series for series for hydrogen like in x, find x.
Solutio:- Wavelength of the first of Lyman series for hydrogen atom will be given by the equation
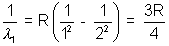
The wavelength of the second Balmen line for hydrogen like i an X is
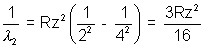
Given
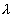 1 =
1 = 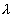 2 or
2 or 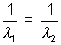
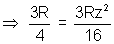
Z = 2
i.e. x ian is He+
8. The p.d. across an X-ray tube is 100000 volts and the current through it is 2.5 mA. Calculate (a) the number of electrons striking the anode per second, (b) the speed with which they strike.
Solution :- (i)
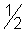 mv2 = e.V => v =
mv2 = e.V => v = 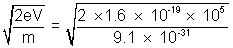 = 1.88 x 108 m/s.
= 1.88 x 108 m/s.(ii) Number of electrons hitting the target per second =
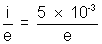
energy incident per second on the target = n.eV =
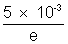 = 5 x 102 J/S
= 5 x 102 J/SHe at produced = 500 x 0.999 = 4.99.5 J/S
MEDIUM
1. A hydrogen like atom (atomic number Z) is in a higher axcited state of quantum number n. The excited atom can make a transition to the second excited state by successively emitting two photos of energies 4.25 eV and 5.95 eV respectively. Determine the values of n and Z.
Solution:- From the given conditions
En - E2 = (10.2 + 17)eV = 27.2 eV ........................................ (i)
En - E3 = (4.25 + 5.95)eV = 10.2 eV ...................................... (ii)
from (i) and (ii) we get
E3 - E2 = 17.0
or Z2 - 13.6
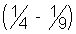 = 17.0
= 17.0=> Z = 3
from equation (i) Z2(13.6)
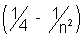 = 27.2
= 27.2Substituting Z = 3 we get, n = 6
 Z = 3 and n = 6
Z = 3 and n = 6