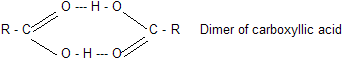Carboxyllic-Acid-1
Carboxylic Acid
Some important acids and their structural formula’s.
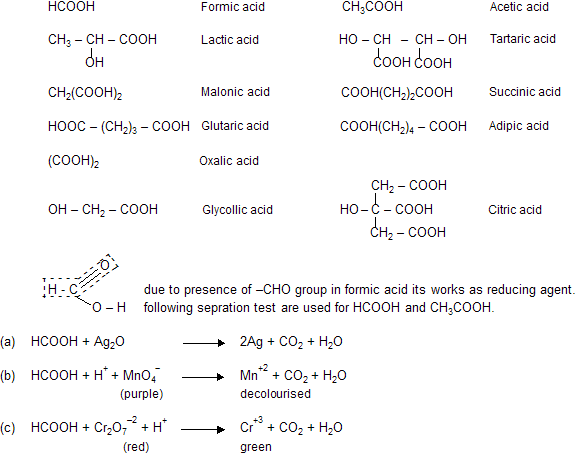
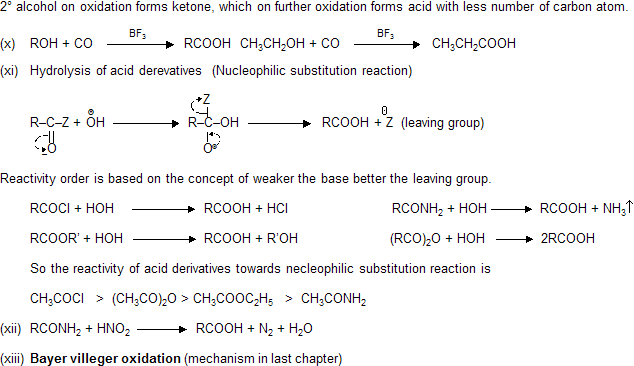
Preparation
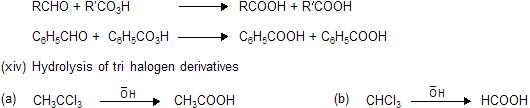
Manufacture or industrial preparation.
| (1) HCOOH (Formic acid) | (2) (COOH)2 Oxallic acid |
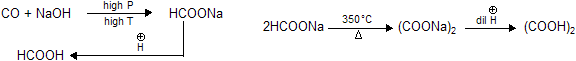
(3) CH3COOH (Acetic acid)
| (b) From pyroligneous acid (already discussed in alcohols). | |
| (c) Quick vinegar process or fermentation process | |
| In the ‘quick vinegar process’, beech shavings, contained in barrels, are moistened with strong vinegar containing the bacteria. A 10% aqueous solution of ethanol containing phosphates and inorganic salts (which are necessary for the fermentation) is then poured through the barrels, and the ethanol is there by oxidised to acetic acid. A plentiful supply of air is necessary, otherwise the oxidation is incomplete and acetaldehyde is produced. | |
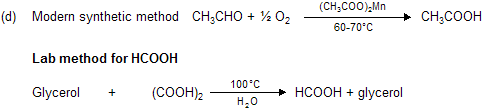 |
The distillate contains formic acid and water. The aqueous formic acid solution cannot be fractionated to give anhydrous formic acid because the boiling point of the acid is 100.5°C. The procedure adopted is to neutralise the aqueous acid solution with lead carbonate, and concentrate the solution until lead formate crystallises out. The precipitate is then recrystallised, dried, and heated at 100°C in a current of hydrogen sulphide.
The anhydrous formic acid which distils over contains a small amount of hydrogen sulphide, and may be freed from the latter by adding some dry lead formate and redistilling. This procedure for obtaining the anhydrous acid from its aqueous solution can only be used for volatile acids.
Physical Properties
As we would from their structure, carboxylic acid molecules are polar, and like alcohol molecules, can form hydrogen bonds with each other and with other kinds of molecules. The aliphatic acids therefore show very much the same solubility behavior as the alcohols. The first four are miscible with water, the five carbon acid is partly soluble, and the higher acids are virtually insoluble. Water solubility undoubtedly arises from hydrogen bonding between the carboxylic acid and water. The simplest aromatic acid, benzoic acid, contains too many carbon atoms to show appreciable solubility in water.
Carboxylic acids are soluble in less polar solvents like ether, alcohol, benzene, etc.
Carboxylic acids are even higher boiling than alcohols. For example, propionic acid (b.p.141°C) boils more than 20°C higher than the alcohol of comparable molecular weight, n-butyl alcohol (b.p. 118°C). These very high boiling points are due to the fact that a pair of carboxylic acid molecules are held together not by one but by two hydrogen bonds.