Carboxyllic-Acid-4
Maleic acid is stronger than fumaric acid due to stable intermediate by intramolecular H-Bonding. Ka1 for maleic acid is higher than fumaric acid but Ka2 is Low because second proton is busy in hydrogen bonding. Here we create a great concept that hydrogen decreases acidic nature but when number of proton are two or more than two it may be increases acidic nature by intermediate stabilization.
(xi) (COOH)2 > HCOOH > CH3COOH
(xii) (COOH)2 > CH2(COOH)2 > (CH2)2(COOH)2
Test’s of Carboxylic acids
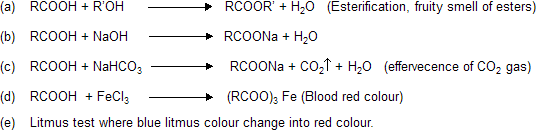
Separation Test’s
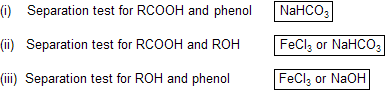
Chemical properties
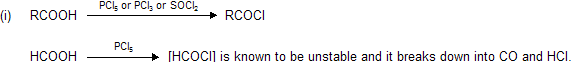
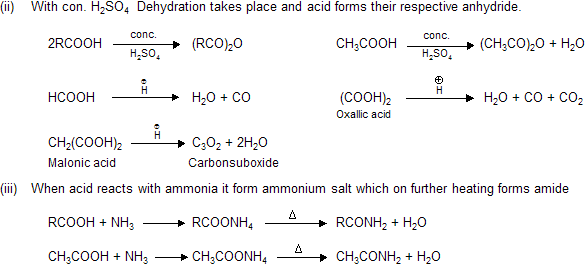
(iv) Effect of heat
When acid undergo heating process they follow ‘Leblanc’ stability rule, for example when single carbon contains two carboxylic group on heating forms mono carboxylic acid with evolution of CO2 gas.
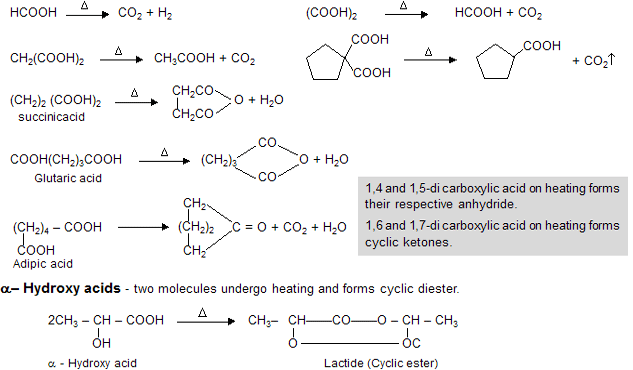
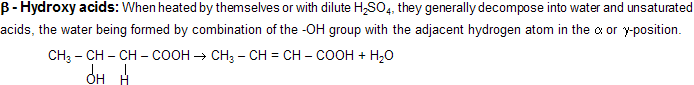
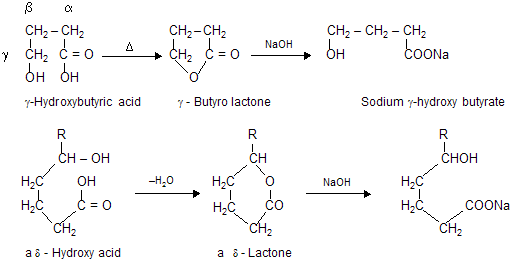
A hydroxy acid is both alcohol and acid. In those cases where a five or six membered ring can be formed, intramolecular esterification occurs. Thus ![]() readily eliminate water to form a cyclic ester known as lactone. The ring readily opens up on treatment with a base to give the salt of the acid.
readily eliminate water to form a cyclic ester known as lactone. The ring readily opens up on treatment with a base to give the salt of the acid.