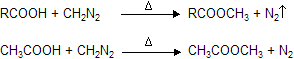Carboxyllic-Acid-6
(xi) Kolbe’s electrolysis
When sodium or potassium salt of acids undergo electrolysis to form alkanes by ionic cum free radical mechanism.
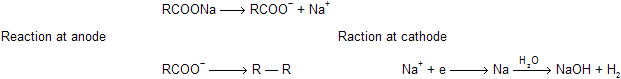
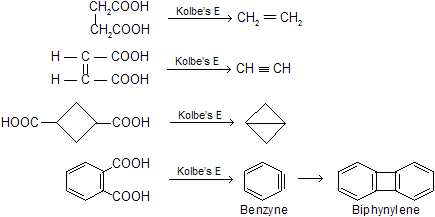
(xii) Decarboxylation by sodalime
Sodalime is a mixture of sodium hydroxide and CaO (CaO is porous in nature absorb CO2 gas due to basic nature) when it reacts with carboxylic acid, forms alkanes with less number of carbon atom, this reaction follows carbanian active mechanism for example
Mechanism of reaction
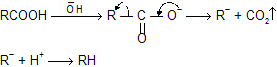
Intermediate carbanian formation takes place so reactivity based on intermediate stability, rate for decarboxylation in following examples.
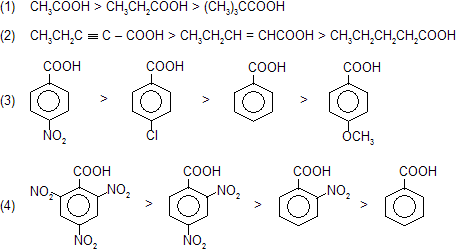
Due to high stability of intermediate 2,4,6-trinitrobenzoic acid liberate CO2 gas even on heating.
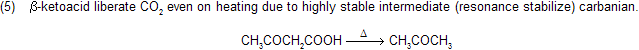
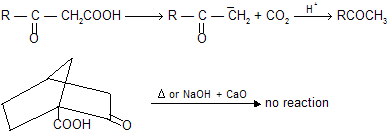
Bridge ![]() donot undergo decarboxylation by sodalime or heating because it form bridge head carbanian which is highly unstable. Carbanian is sp2 hybridised planar structure so it tends to convert nonplanar molecule in to planar structure.
donot undergo decarboxylation by sodalime or heating because it form bridge head carbanian which is highly unstable. Carbanian is sp2 hybridised planar structure so it tends to convert nonplanar molecule in to planar structure.
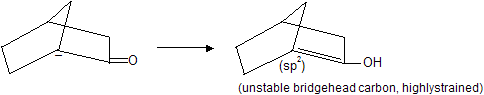
Similarly bridge halide donot undergo elimination reaction easily due to molecular strain (bridge head planar carbanian)
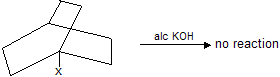
(xiii) Reaction with CH2N2 (Diazomethane)