Chemical - Bonding-3
(3) Solubility. Alcohols, glycol, glycerol and sugars are soluble in water due to the formation of hydrogen bond with water molecules. Dimethylether, (CH3)2O, is miscible in water as it can form hydrogen bond with water molecule but dimethyl sulphide, (CH3)2S, is immiscible as it cannot form hydrogen bond with water molecules since the electronegativity
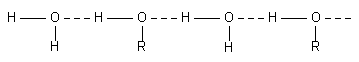
of sulphur is less.
(4) Viscosity. Viscosity increases with the extent of hydrogen bonding in molecules. The viscosity of water is 10.05 millipoise, methanol 6 millipoise and dimethyl ether 2.3 millipoise. Since both H2O and CH3OH are hydrogen bonded the viscosities are high, but when there is substitution of second methyl group to produce the non-hydrogen-bonded dimethyl-ether, (CH3)2O, the viscosity drops to a low value. Polyhydroxy alcohols such as ethylene glycol, CH2OH.CH2OH and glycerol, CH2OH.CHOH.CH2OH which have extensive hydrogen bonding exhibit much higher viscosities.
(5) Molecular weights. The association of two or more molecules by intermolecular hydrogen bonding affect the apparent molecular weight. In case of carboxylic acids (RCOOH) it is observed that the apparent molecular weights are
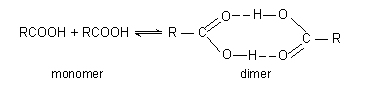
higher than the formula weights. The apparent molecular weight decreases with increasing temperature due to dissociation of dimer into monomer.
A monomer-dimer hydrogen-bonded equilibrium is the simplest explanation of these results. Increase in temperature increases the average kinetic energy of the molecules, breaking more hydrogen bonds and shifting the equilibrium to left.
(6) Dielectric constants and Dipole moments
The formation of hydrogen-bond, A -- H...B leads to an increased polarity of the bond A -- H, and hence, to a larger dielectric constant and greater dipole moment.

(7) Low density of ice than water
In the crystal structure of ice the oxygen atom of water is surrounded by four hydrogen atoms, two attached with covalent bonds and two with hydrogen bonds. Thus in ice every water molecule is associated with four other water molecules in tetrahedral pattern. Ice has an open structure with large empty space due to existence of hydrogen-bonds. When ice melts a number of hydrogen bonds are broken and the space between water molecules decreases and the density of water increases, therefore from 0° to 4°C, it is maximum. Above 4°C the increase in kinetic energy of the molecules disperse them and the result is that the density now decreases with increasing temperature.
(8) Stability of unusual structures
Generally, the organic compounds with two -- OH groups on the same carbon atom are unstable and soon liberate water molecule. For example :
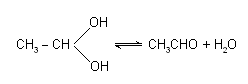
The stability of compounds like chloral hydrate, CCl3 -- CH(OH)2 can be explained on the basis of intramolecular hydrogen-bonding.
(9) Chain, Sheet and Three dimensional structures
Hydrogen bonding leads to the formation of chains (HCN, HF, HCOOH), sheet (orthoboric acid, oxyde acid) and three dimensional network (water, KH2PO4) structures.
(10) Dissociation constants of carboxylic acids
The dissociation constant of an acid depends on the stability of its ion. If the stability of anion of an acid is increased due to intramolecular hydrogen-bonding, the acid strength is greatly enhanced i.e., pKa value decreases. The carboxylate ion of o-hydroxy benzoic acid is stabilised by intermolecular hydrogen-bonding, thus o-salicylic acid is more stronger (pKa = 2.89) than benzoic acid (pKa = 4.17).
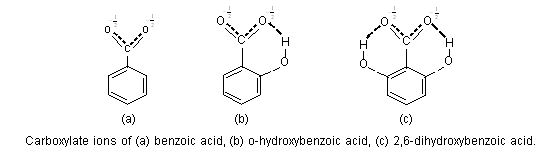
It can be seen that two hydrogen-bonds would be expected to bring more stabilization than one hydrogen bond, and 2,6-dihydroxy benzoic acid is much more stronger (pKa = 2.30) than o-salicylic acid. Similarly, o-salicylic acid (pKa = 2.98) is much stronger due to intramolecular hydrogen bonding than its meta (pKa = 4.08) and para (pKa = 4.58) isomers.

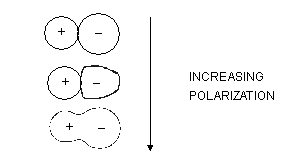
Fajan's Rule
It is generally assumed that covalent and ionic bonds are entirely distinct but this is probably not a totally valid assumption. Bonds intermediate between ionic and covalent do occur through a process of deformation or polarization.
Ionic polarization is favoured by a number of factors which are summarized in the four Fajan's rules.
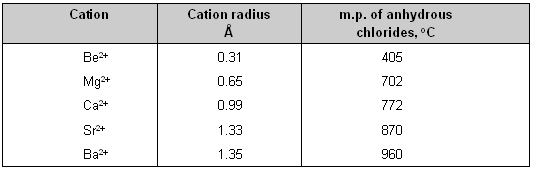
(1) Small cation _ In a small cation there is greater concentration of positive charge over a small surface area so it will cause greater deformation of an anion than would he caused by a large cation. Thus small cations have high polarizing power.
The effect of cationic size upon covalent character is shown in table below. It is clear from the table that with the increase in the size of the cation the covalent character decreases.
This is justified by the low melting point of beryllium chloride which is more covalent than the chlorides of the alkaline earth metals.
Effect of cationic size upon covalent character
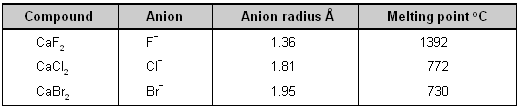
(2) Large anion _ The large anion has polarizability. The outermost orbitals of the anions are shielded from the nucleus by a number of completely occupied orbitals hence they are readily polarized by a small cation.
If the size of the cation and the charge on both the ions is kept constant and only the size of the anion is increased, more covalency will be noticed. This is shown in table from the decrease in the melting points of calcium halides.
Effect of anion size upon covalent character
(3) Large charge on either of the ions_ It is understandable that the electrostatic forces which cause polarization will be considerably increased if the ions are highly charged. The increased nuclear charge will attract the ions to a greater extent causing greater deformation and hence covalent character. The decrease in melting points with the increase of charge of the ions is shown in table to justify this generalization.
Effect of cationic charge upon covalent character
