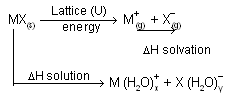Chemical - Bonding-4
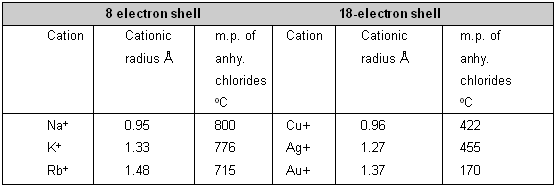
(4) Cation with non-inert gas atom structure _ The cations with the inert gas electron configuration are most effective in shielding the nuclear charge from its surface while the cations with non-inert gas atom structure have positive fields at their surfaces and consequently will possess high polarizing powers. Thus the cation should possess an electronic configuration which is not that of an inert gas.
Hence, if the charge and size are kept nearly constant, cations with 18-electron structure cause greater anion deformation than those with 8-electron arrangements. It is shown in table by the comparison of the melting points of anhydrous chlorides of IA and IB group of periodic table.
Effect of 8 and 18 electronic shell upon the covalent character
On the basis of these important general rules it becomes possible to predict the type of bond that a given element is likely to prefer.
Rules(1), (3) and (4) indicate that the cations which are large and have small charge and possess inert gas electronic configuration should possess least polarizing power e.g., the large alkali metal ions. Thus the large alkali metal ions will prefer to form ionic bond.
Similarly, the small halide ions will favour an ionic bond because they will form ions having the least polarizability. According to rules (2) and (3) the most stable anions are those which are small and have only a small charge.
The fourth Fajan's rule suggests that, in general, the non-transition elements are more ionic than the transition elements because their cations have lower polarizing power and so the cations are more stable.
Applications of Fajan's Rule
(1) Melting point
(2) Diagonal relationship
We know that chemistry of lithium, berylium and boron resembles with that of magnesium, aluminium and silicon, respectively. The diagonal relationship observed between the following pairs of elements can also be explained with the help of Fajan's rules.
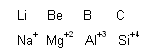
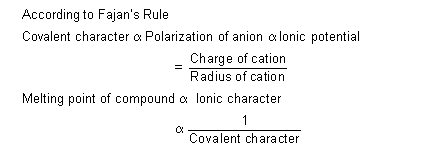
On moving to the right across a period in the periodic table the charge of the cation increases and the size decreases. Consequently' the polarizing power will also increase. In a vertical group, with the increase power of the cation will correspondingly decrease. If both moves are made simultaneously, as in a diagonal relationship then two elements of similar polarizing power may result, e.g., polarizing power of Be2+ and Al3+ is almost similar as their ionic potential (![]() ) are also almost similar [
) are also almost similar [![]() of Be2+ = 6.48 and
of Be2+ = 6.48 and ![]() of Al3+ = 6.0]. Such elements will form bonds of a similar type in the corresponding compounds. This explains almost identical chemical and physical properties of the above mentioned pairs of elements.
of Al3+ = 6.0]. Such elements will form bonds of a similar type in the corresponding compounds. This explains almost identical chemical and physical properties of the above mentioned pairs of elements.
(3) Non- polar character and colour
The increase in nonpolar character of inorganic salts is manifested in the appearance or enhancement of colour. Thus:
Colour deepening tendency ![]() polarization of anion
polarization of anion ![]() size of anion
size of anion
(i) The oxides of colourless cations are usually white but the corresponding sulphides are likely to be deeply coloured if the cation is one which has a tendency to polarize anions. With a few exceptions, the white metal sulphides are only those of alkali and alkaline earth metals.
(ii) In a series of halides of ions such as Ag+, the fluorides and chlorides are colourless ions is usually an indication of an appreciable amount of nonpolar character or some other unusual structural feature. An appreciable amount of polarization leads to intense absorption bands.
(4) Solubility
Solubility of salts in polar solvents like water is affected by polarization. The example of silver halides may be considered in which there is polarizing cation and increasing polarizable anions.
Silver fluoride is quite ionic and soluble in water. Less ionic silver chloride is soluble only after complexation with ammonia. silver bromide and silver iodide are insoluble even with the addition of ammonia. Increasing covalency from fluoride to iodide is expected and decreases solubility in water is observed. However, many other factors are involved in solubility in addition to covalency.
(5) Chemical reactions_ Stability of metal carbonates
Chemical reactions can often be correlated in terms of the polarizing power of a particular cation. For example, in alkaline earth carbonates, there is a tendency towards decomposition with the evolution of carbon dioxide.
With the increase in ionic potential of metal ion [charge/radius], its tendency to accept oxide (O2_) to form metal oxide increases. Hence, the stability of metal carbonates increases down the group in periodic table, radius of the metal ion increases, ionic potential decreases and stability of the metal carbonate increases as shown in table.
![]()
Along the period (from left to right) charge on the metal ion increases, ionic potential increases and stability of the metal carbonate decreases e.g., stability of K2CO3 > CaCO3 > CuCO3.
Further, the effect of d electrons is also evident. Cd2+ and Pb2+ are approximately of the same size as Ca2+ but both CdCO3 and PbCO3 decosmpose at approximately 350°C.
(6) Acidic, Basic and Amphoteric character of oxides
![]()
If ï < 2.2 metal oxide is basic e.g., MnO, CrO, Na2O, MgO etc.
If ï = 2.2 to 3.2 metal oxide is amphoteric e.g., MnO2, CrO2 etc.
If ï > 3.2 metal oxide is acidic e.g., MnO3, CrO3, Mn2O7 etc.
With the increase in ionic potential of metal ion, polarizing power increases, covalent character of M — O bond increases, bond does not dissociate on hydrolysis and the acidic character increases.
Attempt has also been made to correlate the enthalpy of carbonates, sulphates, nitrates and phosphates with increasing charge and size function of the cation. It may therefore be stated in general that size and charge are important factors governing the polarizing power of ions and consequently, many of their chemical properties.
Results of polarization
Polarization results in increasing covalent character in predominantly ionic bonds which may in turn affect the melting and boiling points of ionic compounds, their decomposition temperature, solubility, colour, chemical reactions etc. Possible correlation of these properties with polarization has been discussed earlier.
Solubility of compounds
Solubility of diffrent compounds in solvents depends upon many fectors. It is very complicated property because many factors control this property simultaneously for example
| (a) Nature of solute | (b) Nature of solvent | (c) Temprature of reaction |
| (d) Pressure | (e) Lattice energy | (f) Solvation energy etc. |
When any solute dissolves in solvent to give the saturated solution, heat evolved or absorbed to the surroundings is known as heat of solution.
Whether the heat of solution is positive or negative depends on the nature of solute and solvent. When any solid are dissolved in water enthalpy of over all reaction is depends upon strength of two energies, that is
(i) Energy required to break down one mole ionic crystal lattice into their respective ions known as lattice energy.
(ii) Energy liberated when the ions are solvated or hydrated by solvent there is process of neutralisation, known as solvation energy or hydration energy. This energy actually takes into account of both solvent. Solvent interaction (energy required to make a hole in water) and solvent solute interaction. These two are combined together because experimentally they are hard to seprate. Energy change involved in dissolution of a salt represented by born haber cycle.
In above process U is the lattice energy of the crystal, DH solvation is the energy liberated when positive and negative ions gets solvated and DH solution is the observed heat of solution at in finite dilution. The total energy change from MX(s) to their respective solvated ions is actually independent of the path. Above representation of ionic compound in water is born-Haber type cycle. In case of KCl, overall process can be imagined to occur in two consecutive steps
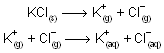
First step involves vapourizing solid requires energy i.e., work must be done to seprate the ions while second step is exothermic because process of solvation where ion-dipole attraction liberate energy. When solvation or hydration energy is greater than the lattice energy the overall process is exothermic so liberation of energy takes place, normally solute is soluble in solvent while if lattice energy is more than hydration energy, process is endothermic and its difficult for solubility of compound in solvent.
Factors affecting Hydration (solvation) energy
(a) Size of ions-
As the size of ions decreases, more in teraction between ions and H2O takes place so hydration energy increases. For example
hydration energy of:
In transition series (d-Block) size of ions are almost same due to balancing of shielding effect and nuclear attraction force, therefore their hydration energy is almost constant. Transition metal ion can form strong bond with water due to presence of vacant d-orbital so they contain high solvation energy.
(b) Charge on ions _ As the charge on ions increases, attraction between ions and dipole (H2O) increases so hydration energy increases.
(c) Dielectric constant of solvent — Measurement of the tendency to attract solute particals by solvent is known as DEC. As the DEC increases force of attraction between ions decreases so solvent with high DEC is responsible for solubility of compound.
Factors affecting Lattice energy
(a) Size of ions — As the size of ions decreases their is compact packing of atoms so system is stable with high lattice energy for example LiF contains high lattice energy.
(b) Charge on ions — As the charge on ions increases, their is high attraction between ions so stability increases which increases lattice energy for example Al2O3 > Mgcl2 > NaCl.
(c) Bond character _ Ionic compounds have high lattice energy than covalent compound due to more stability.
Factors affecting solubility of ionic compounds
Solubility of compounds mainly control by solvation energy and lattice energy with the support of some minor factor like DEC of solvent, hydrogen bonding etc but it is very difficult to set a trend in periodic table for solubility of different compounds because both solvation-energy and lattice energy decreases with increase in size, thing is which decreases more with respect to other, actually this decides the solubility of compound.
Generally, ionic substances dissolve more readily in solvents composed of molecules containing electrostatic dipoles.
Solids are usually crystalline in nature. In the crystal lattice the ions are arranged in a symmetrical fashion and are held in their relative positions by strong electrostatic forces resulting from the charge upon the ions. To breakdown the arrangement of ions in the crystal, these forces must be overcome. Thus, for a substance to be readily soluble, more energy must be provided for the separation of the ions from the crystal than was liberated in building up the ionic lattice. In other words, the energy of solvation must be of greater magnitude than the lattice energy. (The interaction that takes place when a substance is introduced into a solvent is called solvation and the energy change involved in this process is known as the solvation energy).
Thus, both the solvation energy and the lattice energy affect solubility of ionic compounds but in an opposite manner. The important factors affecting solubility of ionic compounds are discussed below.
1. Nature of solvents. As the DEC of solvents increases solubility of ionic compounds increases because it weakens the force of attraction between ions. Force of attraction between ions given by formula
![]()
DEC of water is very high (81), it is the best solvent for ionic compounds. Hydrogen peroxide has higher DEC (92) than water but it not used as a solvent for ionic compounds because it undergo decomposition at room temprature so work as pwerful oxidising agent.
H2O2 H2O + [O]
Nonpolar solvents like benzene, ether and CCl4 fail to solvate the ions so these are not use to dissolve ionic compounds.
2. Size of ions. Both lattice energy and solvation energy depends upon size of ions on following manner
![]()
where r+ and r- are radii of cation and anion. It is clear from the above relations that decrease in the size of the ions affect the two energies in a similar manner. However, the two energies are influenced to different extents and the predominating energy affects the solubility to different extents.
In case of compounds containing large anions e.g., I-, SO42-, CO32-, PO43- etc., the solubility will decrease with increase of cationic size. For example, in case of sulphates of alkaline earth metals, the decrease in solvation energy is more rapid than the decrease in lattice energy with the increase in the size of cation. The solubility of alkaline earth metal sulphates decreases in the following order :
Similarly, the solubility of alkali metal iodides decrease in the following order :
In case of compounds containing small anions e.g., F—, the decrease in lattice energy is more rapid than the decrease in solvation energy with increasing size of the cation. Thus the solubility of the fluorides of alkai metals increase as the size of cation increases as given below :
Solubility of II group carbonates in H2O
BeCO3 > MgCO3 > CaCO3 > SrCO3 > BaCO3 (Large size anion)
BeF2 > BaF2 > SrF2 > CaF2 > MgF2
(Small size anion but exceptionally high solubility of BeF2 due to high hydration energy of Be+2)
Ba(OH)2 > Ca(OH)2 > Mg(OH)2 > Be(OH)2 (Small size anion)
Solubility in water
CaI2 > CaBr2 > CaCl2 > CaF2
(Due to decrease in lattice energy by increase in size)
3. Ionic Charge. With the increasing ionic charge, the lattice energy increases much more rapidly than the solvation energy. Thus, solubility of ionic compounds decreases very sharply as the ionic charge increases.
Effect of Ionic charge on Solubility 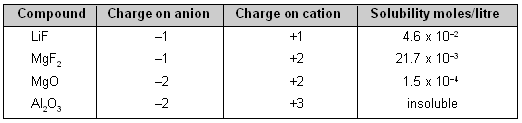
4. Polarization of Anions. Large anions are more polarizable than small anions (Fajan's Rule). Polarization of anion increases covalent character in the molecule, hence decreases solublility in water. This explains the order of solubility of silver halides in water as given below :
AgF > AgCl > AgBr > AgI
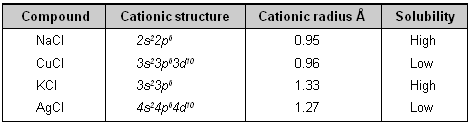
Cations with 18-electrons structure polarize anions more than those with 8-electron arrangements. Polarization of anion decreases the solubility. This explains the following order of solubility
KCl > AgCl
NaCl > CuCl
Compound Cationic structure Cationic radius Å Solubility