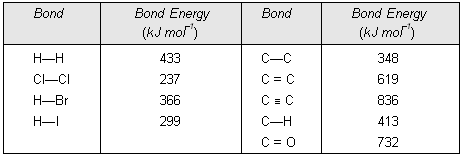Chemical - Bonding-5: sigma bond and pi bond difference
5. Effect of Temperature. Formation of a solution may be exothermic or endothermic process and may be represented as follows :
Exothermic : Solute + Solve Solution + Heat ....(1)
Endothermic : Solute + Solvent + Heat Solution ....(2)
The addition of heat (i.e., a rise in temperature) in equation (2) causes more of the solute to dissolve. This is the case with most of the solid- liquid solutions, where the solubility increases with rise in temperature. In equation (1) the solublility decreases with the rise in temperature. For example, the solubilities of KNO3, NaNO3, KCl, NH4Cl etc. increases with the rise in temperature whereas that of Na2SO4 decreases with the rise in temperature.
6. Hydrogen bonding. It is very minor factor which control solubility of compounds. In the system where solute and solvent particals shows association with H-Bonding are more soluble than other combination. For example NH3 is more soluble in H2O than PH3, ROH are more soluble in H2O than RSH due to association. In case of ROH & ROR their solubility in water is almost same because both show H-Bonding with H2O. Diols and triols are much more soluble than mono hydroxyderivatives because they form more effective hydrogen bonding.
(1) Types of Covalent Bonds
It has already been discussed that the formation of a covalent bond involves the overlapping of half-filled atomic orbitals. The covalent bonds can be classified into two different categories depending upon the type of overlapping. These are :
(a) Sigma covalent bond (b) Pi covalent bond.
(a) Sigma (![]() ) bond. This type of covalent bond is formed by the axial overlapping of half-filled atomic orbitals. The atomic orbitals overlap along the inter-nuclear axis and involve end to end or head on overlap. The electron cloud formed as a result of axial overlap is cylindrically symmetrical about inter-nuclear axis. The electrons constituting sigma bond are called sigma electrons. There can be three types of axial overlap as discussed below :
) bond. This type of covalent bond is formed by the axial overlapping of half-filled atomic orbitals. The atomic orbitals overlap along the inter-nuclear axis and involve end to end or head on overlap. The electron cloud formed as a result of axial overlap is cylindrically symmetrical about inter-nuclear axis. The electrons constituting sigma bond are called sigma electrons. There can be three types of axial overlap as discussed below :
(i) s-s overlap. It involves mutual overlap of half-filled s-orbitals of the atoms approaching to form a bond. The bond formed is called s-s ![]() bond.
bond.
(ii) s-p overlap. It involves mutual overlap of half-filled s-orbital of the one atom with half-filled p-orbital of the other. The bond so formed is called s-p ![]() bond.
bond.
(iii) p-p overlap. It involves mutual overlap of half-filled p-orbitals of the two atoms. The bond so formed is called p-p ![]() bond.
bond.
The s-s, s-p and p-p overlaps have been shown diagramatically in Figure below.

Strength of three types of sigma bonds. The strength of three types of sigma bonds varies as follows :
p-p > p-s > s-s
It is because of the fact, that p-orbitals allow overlap to a greater extent as compared to p-s which is larger as compared to s-s overlap.
(b) Pi (![]() ) Bond. This type of covalent bond is formed by the lateral or sidewise overlap of the atomic orbitals. The orbitals overlap takes place in such a way that their axes are parallel to each other but perpendicular to the internuclear axis. The pi bond consists of two charge clouds above and below the plane of the atoms involved in the bond formation. The electrons involved in the p-bond formation are called
) Bond. This type of covalent bond is formed by the lateral or sidewise overlap of the atomic orbitals. The orbitals overlap takes place in such a way that their axes are parallel to each other but perpendicular to the internuclear axis. The pi bond consists of two charge clouds above and below the plane of the atoms involved in the bond formation. The electrons involved in the p-bond formation are called ![]() -electrons.
-electrons.
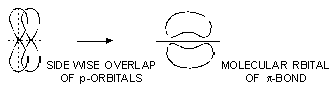
It may be noted that :
(i) Sigma bond is stronger than pi bond. It is because of the fact that overlapping of atomic orbitals can take place to a greater extent during the formation of sigma bond whereas overlapping of orbitals occurs to a smaller extent during the formation of pi bond.
(ii) Pi bond between the two atoms is formed only in addition to a sigma bond. It is because of the fact that the atoms constituting a single bond prefer to form a strong sigma bond rather than a weak pi bond. Thus, pi bond is always present in molecules having multiple bonds, i.e., double or triple bond. In other words, a single bond cannot be a pi bond.
(iii) The shape of molecule is controlled by the sigma frame work (orientations of sigma bonds) around the central atom. Pi bonds are superimposed on sigma bonds hence they simply modify the dimensions of the molecule.
Compraison between sigma and pi bonds. The various points of distinction between sigma and pi bonds are given in Table below.
Table below. Comparison of Sigma and Pi Bonds
he formation of sigma and pi bonds, in oxygen (O2) molecule.
Oxygen atom (8O) has two half-filled p-orbitals in its valence shell as is evident from its electronic configuration (1s2, 2s2, 2px2, 2py1>, 2pz21). One of the half-filled p-orbital overlaps axially with half-filled p-orbital of the other oxygen atom to form s bond. The other half-filled p-orbitals of the two atoms overlap sidewise to form a ![]() bond which is denoted as p
bond which is denoted as p![]() -p
-p![]() bond. The formation of molecule is shown in Fig. below.
bond. The formation of molecule is shown in Fig. below.
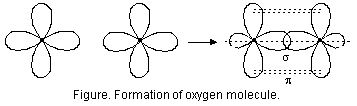
Thus, O = O bond consists of one s bond and one p bond.
Bonding Parameters
Covalent bonds are characterised by the following parameters, bond energy, bond length and bond angle.
(a) Bond Energy
It has already been pointed out that the formation of a bond occurs as a result of decrease of energy. Therefore, same amount of energy is required to break the bond between the two atoms. For example, the energy released during the formation of bonds between the gaseous hydrogen atoms to form one mole of hydrogen moleculs is 433 kJ mol-1. This energy involved in making or breaking of bonds is referred to as bond energy. Thus, bond energy may be defined as the amount of energy required to break one mole of bonds of same kind so as to separate the bonded atoms in the gaseous state.
The magnitude of bond energy reflects the strength of the bond. Its magnitude depends upon the following factors :
(i) Size of the participating atoms. Larger the size of the atoms involved in bond formation, lesser is the extent of overlapping and consequently, smaller is the value of bond energy.
For example, bond energy of Cl—Cl bond is 237 kJ mol-1 whereas that of H—H bond is 433 kJ mol-1.
(ii) Multiplicity of bonds. The magnitude of bond energy increases with the multiplicity of bonds even though the atoms involved in the bond formation are same. It is because of the fact that with the multiplicity of bonds the number of shared electrons between the atoms increases. As a result, the attractive force between nuclei and electrons also increases and consequently, the magnitude of bond energy increases. For example, bond energy of C — C bond is 348 kJ/mol-1 but that of C = C bond is 619 kJ mol-1. The average bond energies of some bonds are given in Table below.
(iii) Number of lone pairs of electrons. Greater the number of lone pair of electrons present on the bonded atoms, greater is the repulsive interactions between them and smaller is the bond energy. Let us compare the bond energies of some of the single bonds
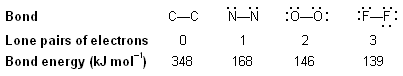
Table below. Bond Energies of Some Common Bonds